Brain Tumor Res Treat.
2021 Oct;9(2):63-69. 10.14791/btrt.2021.9.e16.
Effect of Cadherin-11 Expression on the Prognosis of a Newly Diagnosed Primary Glioblastoma
- Affiliations
-
- 1School of Medicine, Kyungpook National University, Daegu, Korea
- 2Department of Pathology, Keimyung University School of Medicine, Daegu, Korea
- 3Departments of Neurosurgery, School of Medicine, Kyungpook National University, Daegu, Korea
- 4Departments of Pathology, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2522221
- DOI: http://doi.org/10.14791/btrt.2021.9.e16
Abstract
- Background
Cadherin-11, a cell-to-cell adhesion molecule, is associated with higher tumor grade and decreased patient survival. The purpose of this study was to investigate the clinical significance of cadherin-11 expression in the progression and prognosis of a newly diagnosed primary glioblastoma (GBL).
Methods
Between 2007 and 2016, 52 out of 178 patients diagnosed with a GBL and satisfied the following criteria: 1) a new primary GBL, 2) gross-total resection, 3) immunohistochemically-available tissue, and 4) standardized adjuvant treatment.
Results
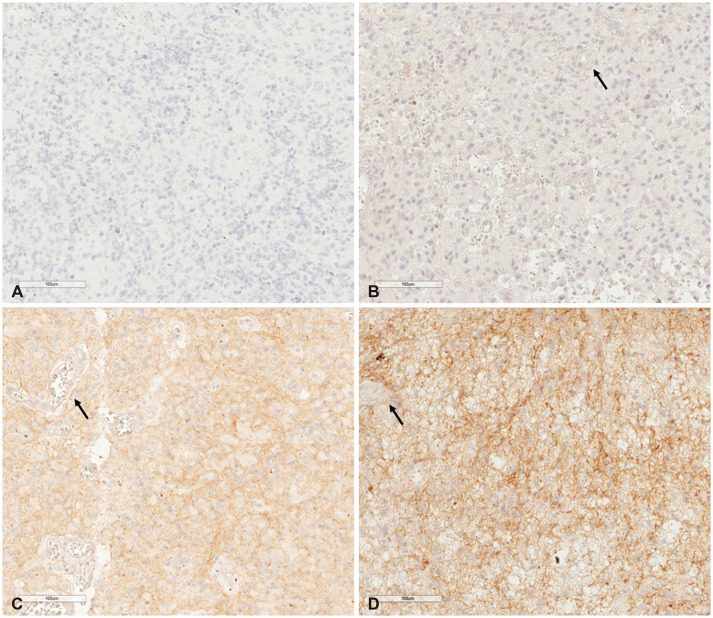
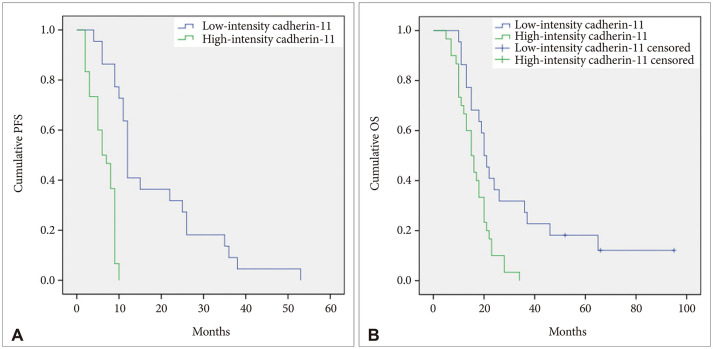
In terms of staining intensity, the low-intensity cadherin-11 group showed longer progression-free survival (PFS) than the high-intensity cadherin-11 group (median PFS, 12.0 months [95% CI, 11.1-12.9] vs. median PFS, 6.0 months [95% CI, 3.7-8.3]; p<0.001). The low-intensity cadherin-11 group revealed longer overall survival (OS) than the high-intensity cadherin-11 group (median OS, 20.0 months [95% CI, 11.8-16.6] vs. median OS, 15.0 months [95% CI, 11.8-18.2]; p=0.003). The staining intensity of cadherin-11 was a statistically significant factor in PFS and OS in terms of univariate and multivariate analyses (univariate analysis: p<0.001 and p=0.005; multivariate analysis: p<0.001 and p=0.005).
Conclusion
Our clinical study demonstrates high cadherin-11 expression may be associated with poor PFS and OS for a newly diagnosed primary GBL.
Keyword
Figure
Reference
-
1. Hanif F, Muzaffar K, Perveen K, Malhi SM, Simjee ShU. Glioblastoma multiforme: a review of its epidemiology and pathogenesis through clinical presentation and treatment. Asian Pac J Cancer Prev. 2017; 18:3–9. PMID: 28239999.2. Hwang JH, Smith CA, Salhia B, Rutka JT. The role of fascin in the migration and invasiveness of malignant glioma cells. Neoplasia. 2008; 10:149–159. PMID: 18283337.3. Roma AA, Prayson RA. Fascin expression in 90 patients with glioblastoma multiforme. Ann Diagn Pathol. 2005; 9:307–311. PMID: 16308158.4. Brady-Kalnay SM, Rimm DL, Tonks NK. Receptor protein tyrosine phosphatase PTPmu associates with cadherins and catenins in vivo. J Cell Biol. 1995; 130:977–986. PMID: 7642713.5. Ireton RC, Davis MA, van Hengel J, et al. A novel role for p120 catenin in E-cadherin function. J Cell Biol. 2002; 159:465–476. PMID: 12427869.6. Davis MA, Ireton RC, Reynolds AB. A core function for p120-catenin in cadherin turnover. J Cell Biol. 2003; 163:525–534. PMID: 14610055.7. Xiao K, Allison DF, Buckley KM, et al. Cellular levels of p120 catenin function as a set point for cadherin expression levels in microvascular endothelial cells. J Cell Biol. 2003; 163:535–545. PMID: 14610056.8. Davis MA, Reynolds AB. Blocked acinar development, E-cadherin reduction, and intraepithelial neoplasia upon ablation of p120-catenin in the mouse salivary gland. Dev Cell. 2006; 10:21–31. PMID: 16399075.9. Bertocchi C, Wang Y, Ravasio A, et al. Nanoscale architecture of cadherin-based cell adhesions. Nat Cell Biol. 2017; 19:28–37. PMID: 27992406.10. Ling K, Bairstow SF, Carbonara C, Turbin DA, Huntsman DG, Anderson RA. Type I gamma phosphatidylinositol phosphate kinase modulates adherens junction and E-cadherin trafficking via a direct interaction with mu 1B adaptin. J Cell Biol. 2007; 176:343–353. PMID: 17261850.11. Anastasiadis PZ. p120-ctn: a nexus for contextual signaling via Rho GTPases. Biochim Biophys Acta. 2007; 1773:34–46. PMID: 17028013.12. Anastasiadis PZ, Moon SY, Thoreson MA, et al. Inhibition of RhoA by p120 catenin. Nat Cell Biol. 2000; 2:637–644. PMID: 10980705.13. Kaur H, Phillips-Mason PJ, Burden-Gulley SM, et al. Cadherin-11, a marker of the mesenchymal phenotype, regulates glioblastoma cell migration and survival in vivo. Mol Cancer Res. 2012; 10:293–304. PMID: 22267545.14. Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005; 352:987–996. PMID: 15758009.15. Wen PY, Macdonald DR, Reardon DA, et al. Updated response assessment criteria for high-grade gliomas: response assessment in neurooncology working group. J Clin Oncol. 2010; 28:1963–1972. PMID: 20231676.16. Pohlodek K, Tan YY, Singer CF, Gschwantler-Kaulich D. Cadherin-11 expression is upregulated in invasive human breast cancer. Oncol Lett. 2016; 12:4393–4398. PMID: 28101202.17. Karsy M, Neil JA, Guan J, Mahan MA, Colman H, Jensen RL. A practical review of prognostic correlations of molecular biomarkers in glioblastoma. Neurosurg Focus. 2015; 38:E4.18. Rubinfeld B, Souza B, Albert I, et al. Association of the APC gene product with beta-catenin. Science. 1993; 262:1731–1734. PMID: 8259518.19. Thomas KR, Capecchi MR. Targeted disruption of the murine int-1 proto-oncogene resulting in severe abnormalities in midbrain and cerebellar development. Nature. 1990; 346:847–850. PMID: 2202907.20. Weis WI. Cadherin structure: a revealing zipper. Structure. 1995; 3:425–427. PMID: 7663938.21. Ozawa M, Engel J, Kemler R. Single amino acid substitutions in one Ca2+ binding site of uvomorulin abolish the adhesive function. Cell. 1990; 63:1033–1038. PMID: 2257621.22. Aberle H, Schwartz H, Kemler R. Cadherin-catenin complex: protein interactions and their implications for cadherin function. J Cell Biochem. 1996; 61:514–523. PMID: 8806074.23. Kemler R. From cadherins to catenins: cytoplasmic protein interactions and regulation of cell adhesion. Trends Genet. 1993; 9:317–321. PMID: 8236461.24. Schulte JD, Srikanth M, Das S, et al. Cadherin-11 regulates motility in normal cortical neural precursors and glioblastoma. PLoS One. 2013; 8:e70962. PMID: 23951053.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Prognostic Significance of E-cadherin Expression in Renal Cell Carcinoma
- Expression of e-cadherin and beta-catenin in relation to clinicopathologic features in oral squamous cell carcinoma
- Loss of E-cadherin Function is Suggested to be Associated with Peritoneal Seeding in Colorectal Cancer
- Reduced Expression of E-cadherin in Human Non-small Cell Lung Carcinoma
- Expression of E-cadherin and a-catenin in Thyroid Carcinomas