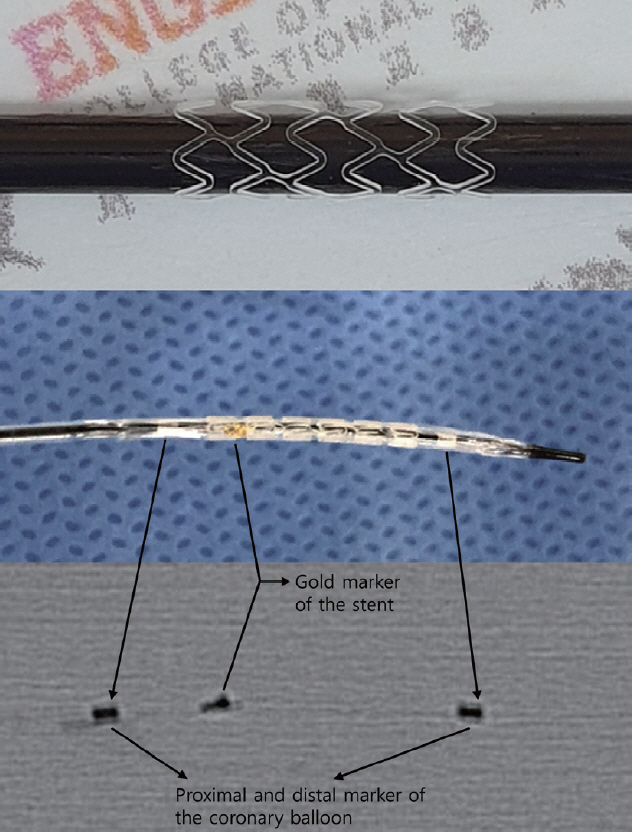
J Korean Neurosurg Soc.
2021 Nov;64(6):853-863. 10.3340/jkns.2021.0009.
Improved Biocompatibility of Intra-Arterial Poly-L-Lactic Acid Stent by Tantalum Ion Implantation : 3-Month Results in a Swine Model
- Affiliations
-
- 1Department of Neurosurgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Department of Materials Science and Engineering, Seoul National University, Seoul, Korea
- 3Department of Pathology, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea
- 4Department of Materials Science and Engineering, Chosun University, Gwangju, Korea
- KMID: 2521975
- DOI: http://doi.org/10.3340/jkns.2021.0009
Abstract
Objective
: Biodegradable poly-L-lactic acid (PLLA) with a highly biocompatible surface via tantalum (Ta) ion implantation can be an innovative solution for the problems associated with current biodegradable stents. The purpose of this study is to develop a Ta-implanted PLLA stent for clinical use and to investigate its biological performance capabilities.
Methods
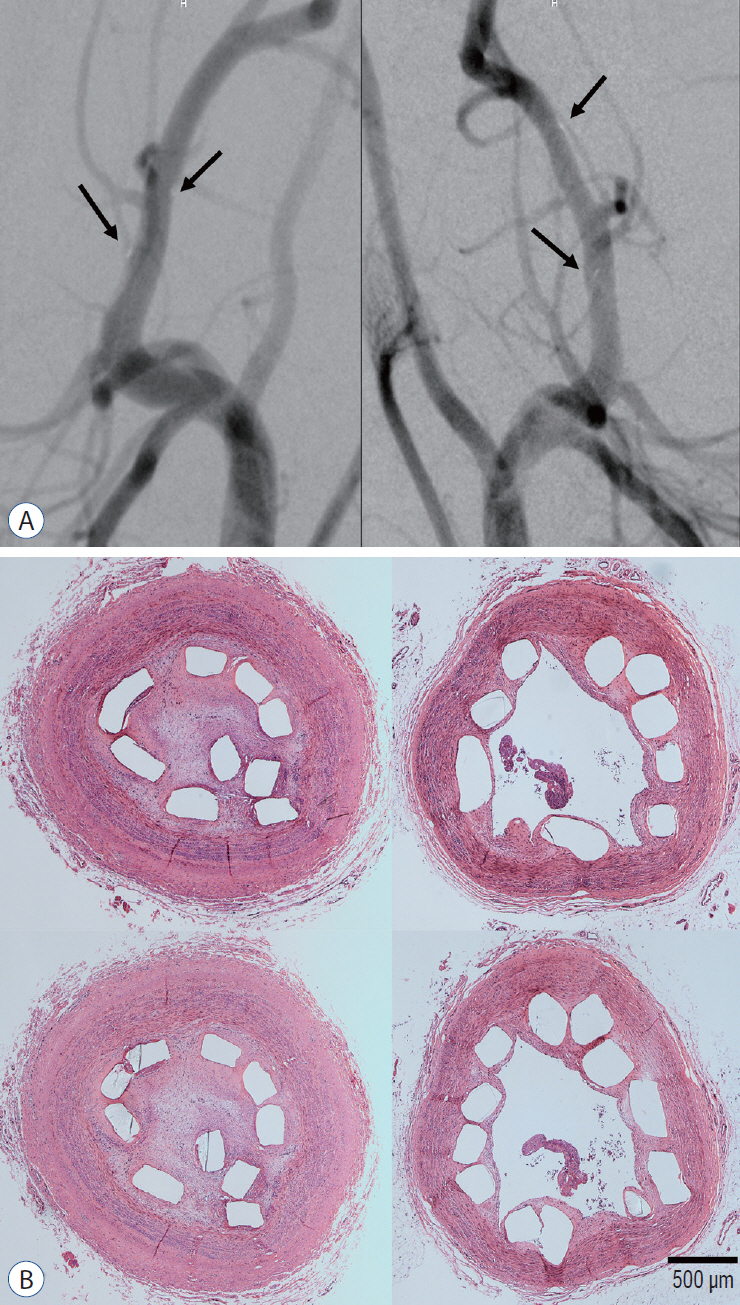
: A series of in vitro and in vivo tests were used to assess the biological performance of bare and Ta-implanted PLLA stents. The re-endothelialization ability and thrombogenicity were examined through in vitro endothelial cell and platelet adhesion tests. An in vivo swine model was used to evaluate the effects of Ta ion implantation on subacute restenosis and thrombosis. Angiographic and histologic evaluations were conducted at one, two and three months post-treatment.
Results
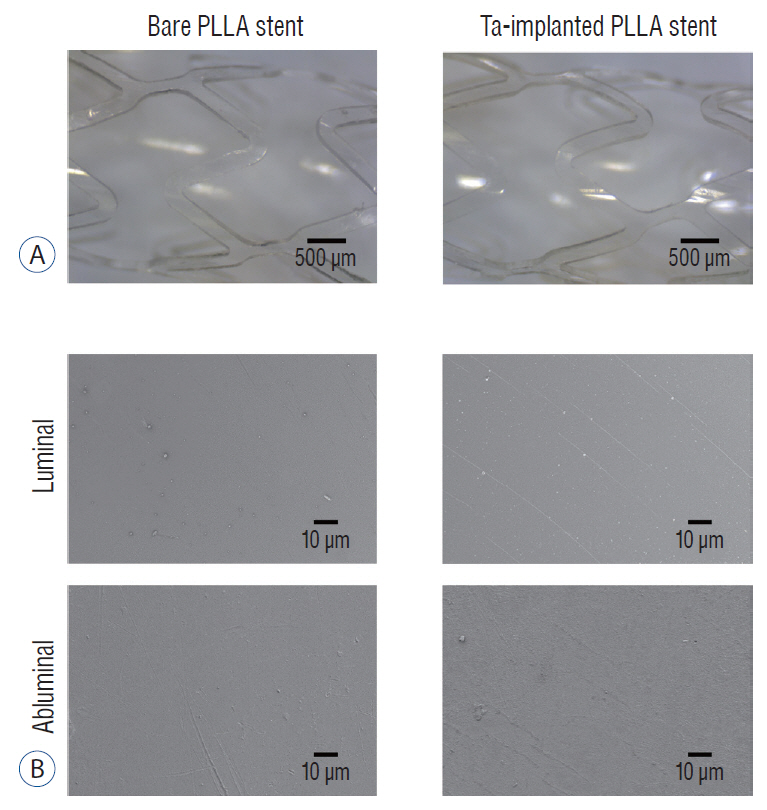
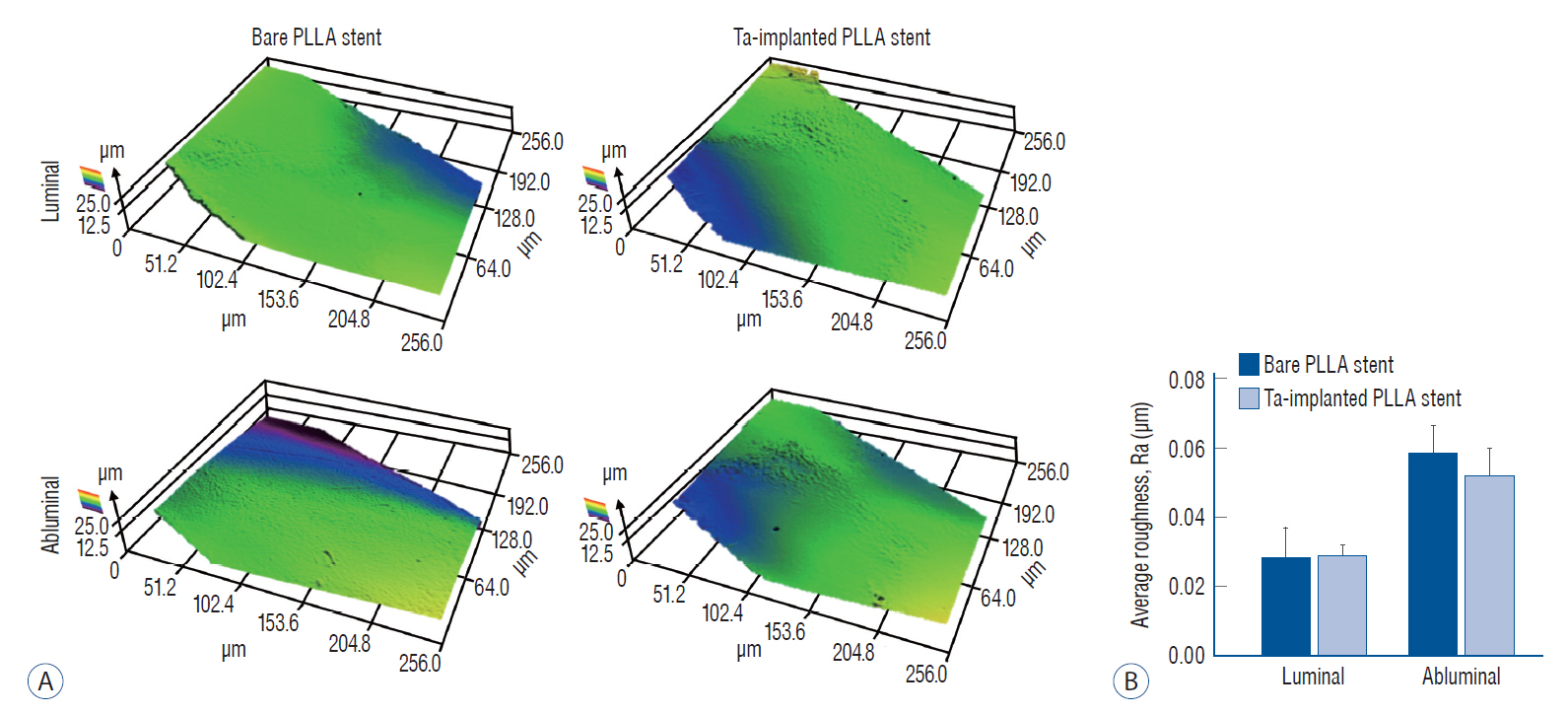
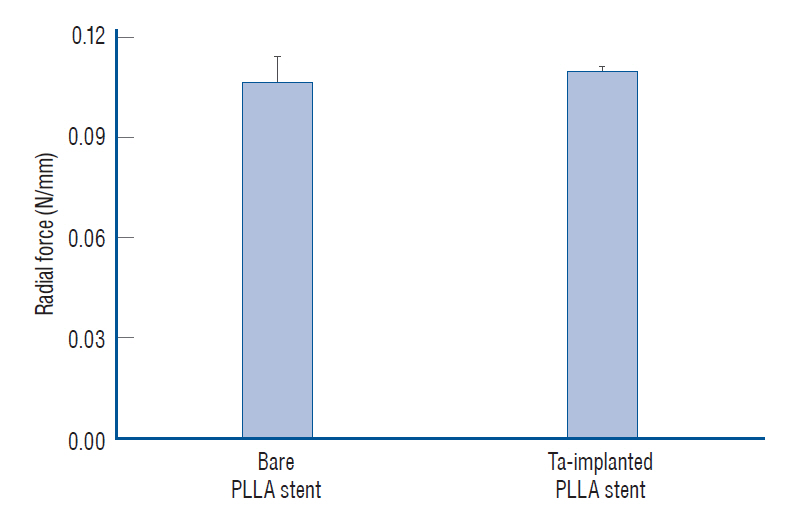
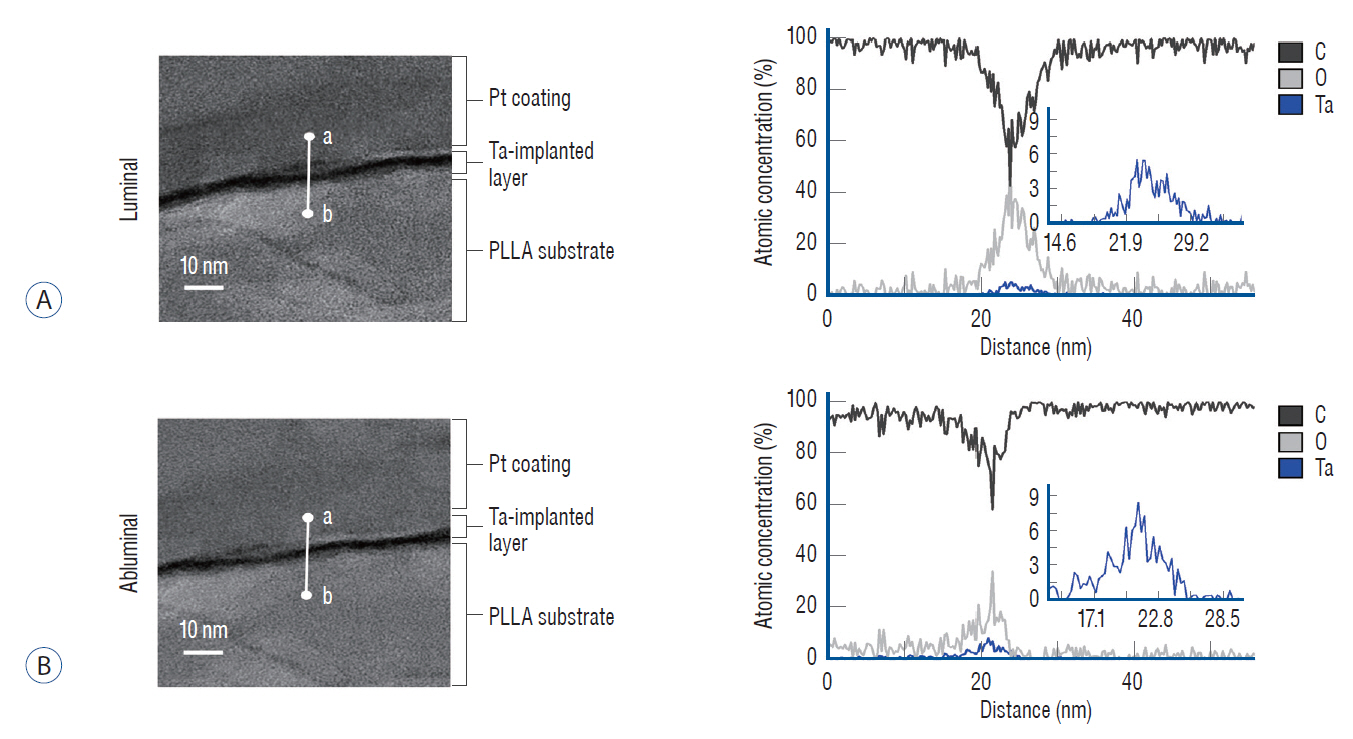
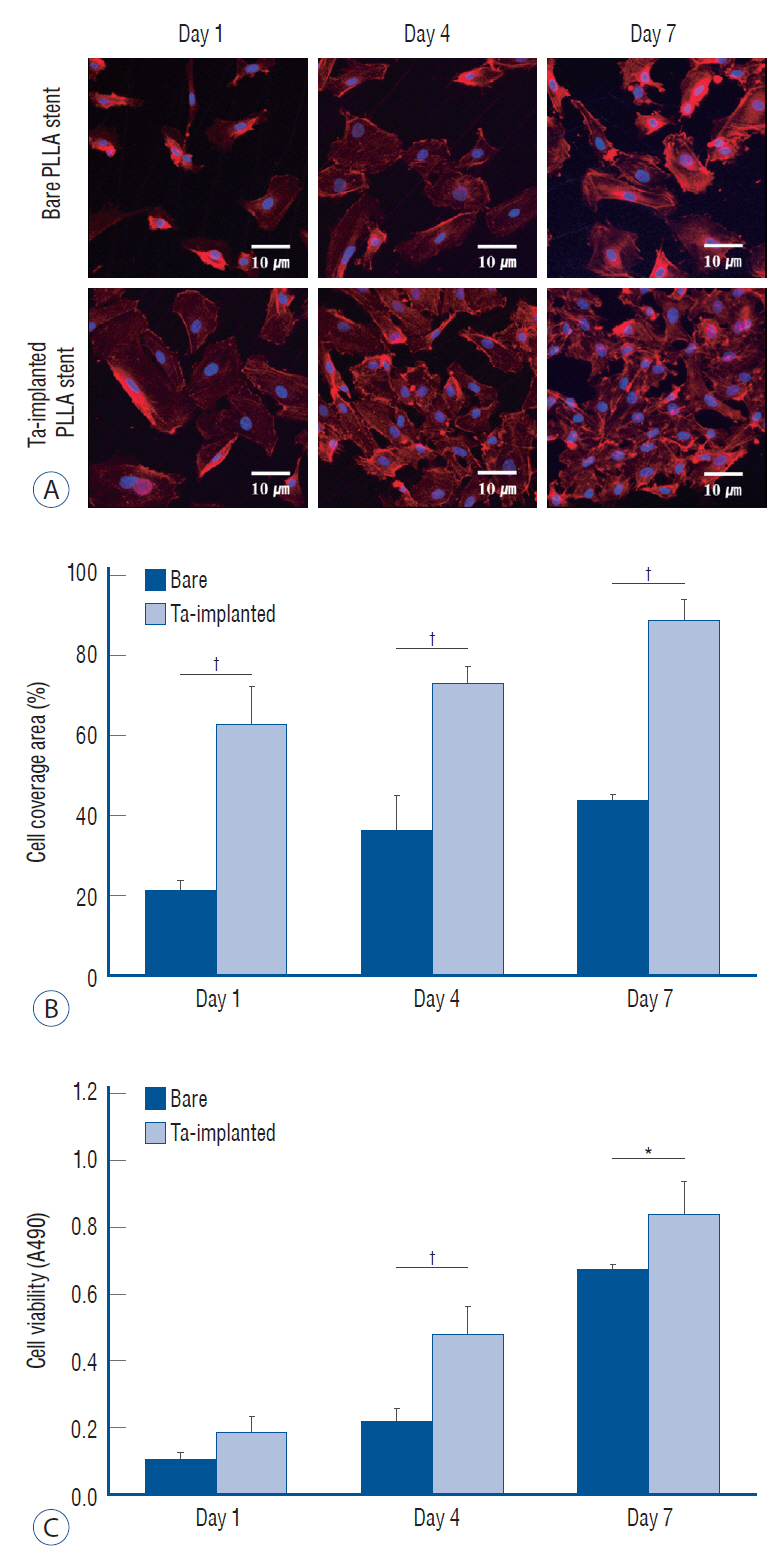
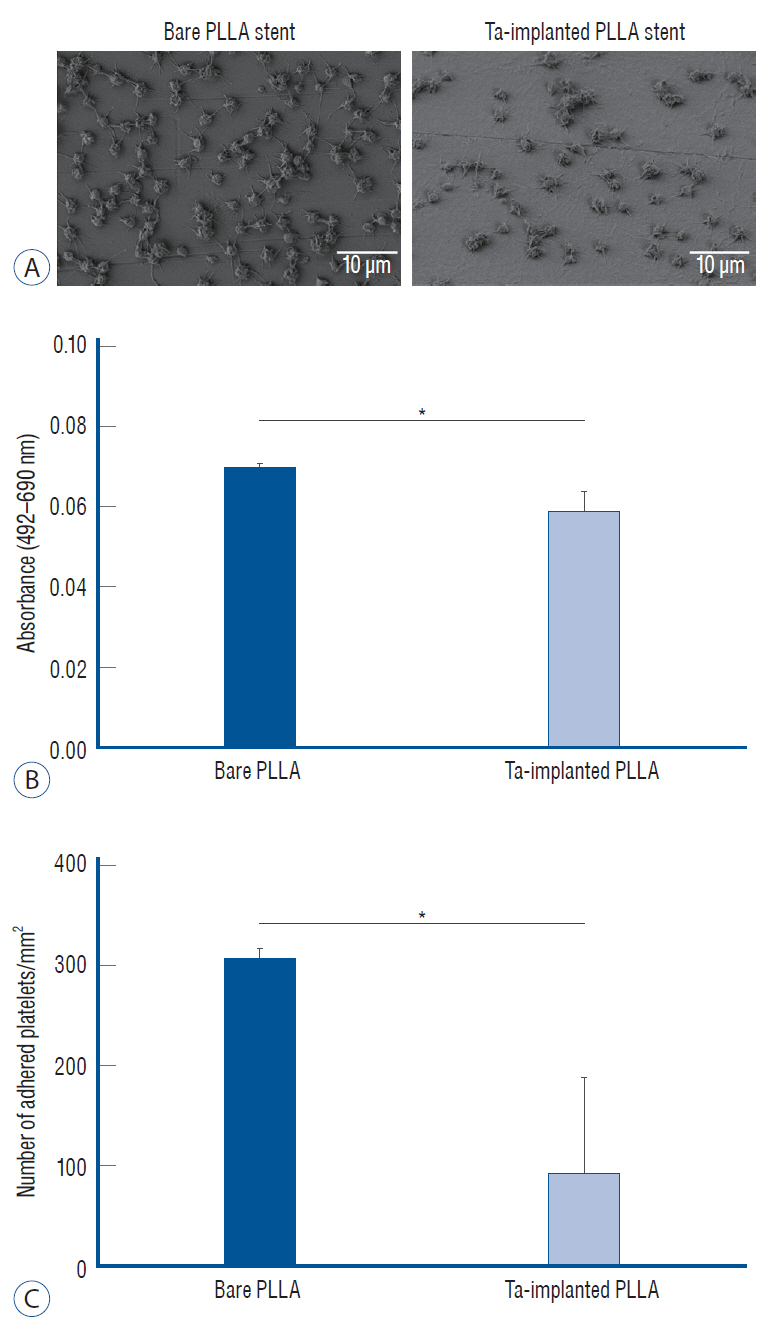
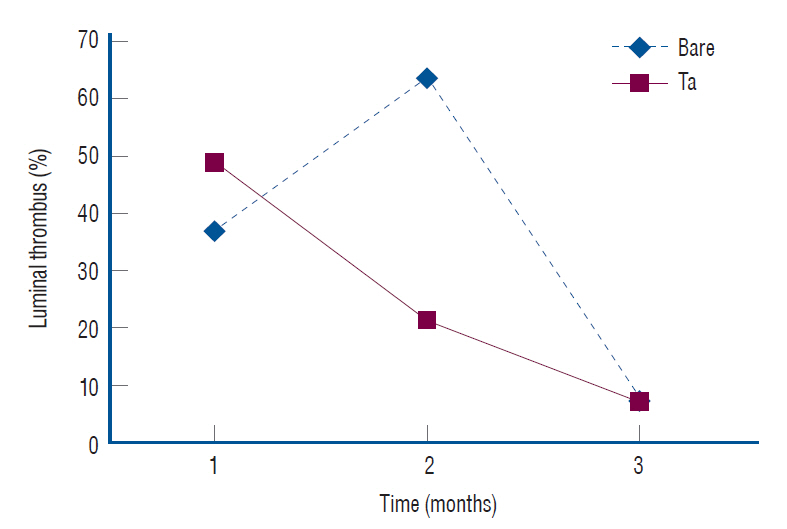
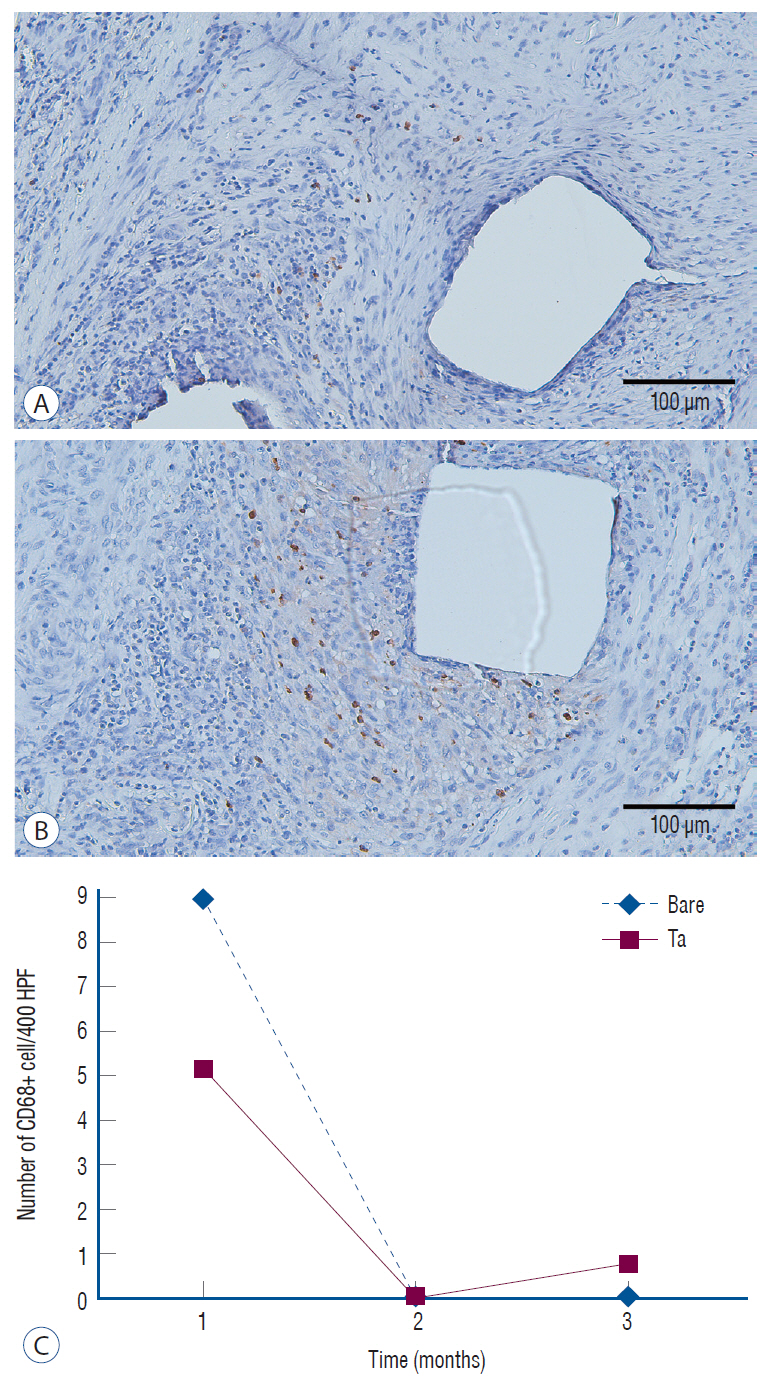
: The Ta-implanted PLLA stent was successfully fabricated, exhibiting a smooth surface morphology and modified layer integration. After Ta ion implantation, the surface properties were more favorable for rapid endothelialization and for less platelet attachment compared to the bare PLLA stent. In an in vivo animal test, follow-up angiography showed no evidence of in-stent stenosis in either group. In a microscopic histologic examination, luminal thrombus formation was significantly suppressed in the Ta-implanted PLLA stent group according to the 2-month follow-up assessment (21.2% vs. 63.9%, p=0.005). Cells positive for CD 68, a marker for the monocyte lineage, were less frequently identified around the Ta-implanted PLLA stent in the 1-month follow-up assessments.
Conclusion
: The use of a Ta-implanted PLLA stent appears to promote re-endothelialization and anti-thrombogenicity.
Keyword
Figure
Reference
-
References
1. Barbarash LS, Bolbasov EN, Antonova LV, Matveeva VG, Velikanova EA, Shesterikov EV, et al. Surface modification of poly-ε-caprolactone electrospun fibrous scaffolds using plasma discharge with sputter deposition of a titanium target. Materials Letters. 171:87–90. 2016.
Article2. Bledzki AK, Jaszkiewicz A, Scherzer D. Mechanical properties of PLA composites with man-made cellulose and abaca fibres. Compos Part A Appl Sci Manuf. 40:404–412. 2009.
Article3. Chen JY, Leng YX, Tian XB, Wang LP, Huang N, Chu PK, et al. Antithrombogenic investigation of surface energy and optical bandgap and hemocompatibility mechanism of Ti(Ta+5)O2 thin films. Biomaterials. 23:2545–2552. 2002.
Article4. Collet C, Asano T, Miyazaki Y, Tenekecioglu E, Katagiri Y, Sotomi Y, et al. Late thrombotic events after bioresorbable scaffold implantation: a systematic review and meta-analysis of randomized clinical trials. Eur Heart J. 38:2559–2566. 2017.
Article5. Hanawa T. Metal ion release from metal implants. Mat Sci Eng C-Bio S. 24:745–752. 2004.
Article6. Hou LD, Li Z, Pan Y, Sabir M, Zheng YF, Li L. A review on biodegradable materials for cardiovascular stent application. Front Mater Sci. 10:238–259. 2016.
Article7. Hu T, Yang C, Lin S, Yu Q, Wang G. Biodegradable stents for coronary artery disease treatment: recent advances and future perspectives. Mater Sci Eng C Mater Biol Appl. 91:163–178. 2018.
Article8. Hwang G, Kim JG, Song KS, Lee YJ, Villavicencio JB, Suroto NS, et al. Delayed ischemic stroke after stent-assisted coil placement in cerebral aneurysm: characteristics and optimal duration of preventative dual antiplatelet therapy. Radiology. 273:194–201. 2014.
Article9. Ikarashi Y, Toyoda K, Ohsawa N, Uchima T, Tsuchiya T, Kaniwa M, et al. Comparative studies by cell culture and in vivo implantation test on the toxicity of natural rubber latex materials. J Biomed Mater Res. 26:339–356. 1992.
Article10. Jin W, Wang G, Lin Z, Feng H, Li W, Peng X, et al. Corrosion resistance and cytocompatibility of tantalum-surface-functionalized biomedical ZK60 Mg alloy. Corros Sci. 114:45–56. 2017.
Article11. Kereiakes DJ, Ellis SG, Metzger C, Caputo RP, Rizik DG, Teirstein PS, et al. 3-year clinical outcomes with everolimus-eluting bioresorbable coronary scaffolds: the ABSORB III trial. J Am Coll Cardiol. 70:2852–2862. 2017.
Article12. Krischek Ö, Miloslavski E, Fischer S, Shrivastava S, Henkes H. A comparison of functional and physical properties of self-expanding intracranial stents [Neuroform3, Wingspan, Solitaire, Leo(+), Enterprise]. Minim Invasive Surg. 54:21–28. 2011.
Article13. Nie FL, Zheng YF, Wang Y, Wang JT. Microstructures, mechanical behavior, cellular response, and hemocompatibility of bulk ultrafine-grained pure tantalum. J Biomed Mater Res B Appl Biomater. 102:221–230. 2014.
Article14. Park C, Seong YJ, Kang IG, Song EH, Lee H, Kim J, et al. Enhanced osseointegration ability of poly(lactic acid) via tantalum sputtering-based plasma immersion ion implantation. ACS Appl Mater Interfaces. 11:10492–10504. 2019.
Article15. Pizzoferrato A, Ciapetti G, Savarino L, Stea S, Tarabusi C. Results of histological grading on 100 cases of hip prosthesis failure. Biomaterials. 9:314–318. 1988.
Article16. Räber L, Brugaletta S, Yamaji K, O’Sullivan CJ, Otsuki S, Koppara T, et al. Very late scaffold thrombosis: intracoronary imaging and histopathological and spectroscopic findings. J Am Coll Cardiol. 66:1901–1914. 2015.17. Rasal RM, Janorkar AV, Hirt DE. Poly(lactic acid) modifications. Prog Polym Sci. 35:338–356. 2010.
Article18. Schwartz RS, Huber KC, Murphy JG, Edwards WD, Camrud AR, Vlietstra RE, et al. Restenosis and the proportional neointimal response to coronary artery injury: results in a porcine model. J Am Coll Cardiol. 19:267–274. 1992.
Article19. Sharkawi T, Cornhill F, Lafont A, Sabaria P, Vert M. Intravascular bioresorbable polymeric stents: a potential alternative to current drug eluting metal stents. J Pharm Sci. 96:2829–2837. 2007.
Article20. Stone GW, Ellis SG, Gori T, Metzger DC, Stein B, Erickson M, et al. Blinded outcomes and angina assessment of coronary bioresorbable scaffolds: 30-day and 1-year results from the ABSORB IV randomised trial. Lancet. 392:1530–1540. 2018.
Article21. Suyatma NE, Copinet A, Tighzert L, Coma V. Mechanical and barrier properties of biodegradable films made from chitosan and poly (lactic acid) blends. J Polym Environ. 12:1–6. 2004.
Article22. Tverdokhlebov SI, Bolbasov EN, Shesterikov EV, Antonova LV, Golovkin AS, Matveeva VG, et al. Modification of polylactic acid surface using RF plasma discharge with sputter deposition of a hydroxyapatite target for increased biocompatibility. Appl Surf Sci. 329:32–39. 2015.
Article23. Wang J, Jin X, Huang Y, Ran X, Luo D, Yang D, et al. Endovascular stent-induced alterations in host artery mechanical environments and their roles in stent restenosis and late thrombosis. Regen Biomater. 5:177–187. 2018.
Article24. Wiebe J, Nef HM, Hamm CW. Current status of bioresorbable scaffolds in the treatment of coronary artery disease. J Am Coll Cardiol. 64:2541–2551. 2014.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Biodegradation and Biocompatibility of Poly L-lactic Acid Implantable Mesh
- Comparison of Slotted Tube versus Coil Stent Implantation for Ostial Left Anterior Descending Coronary Artery Stenosis: Initial and Late Clinical Outcomes
- "Late Clinical Outcomes of Cordis Tantalum Coronary Stenting without Anticoagulation : Validation of Angiographic Measurement and In-stent Restenosis by Intravascular Ultrasound"
- Management of a Visible Nodule Following Poly-L-Lactic Acid Injection in the Periorbital Area
- Effect of Thermoresponsive Poly(L-lactic acid)-poly (ethylene glycol) Gel Injection on Left Ventricular Remodeling in a Rat Myocardial Infarction Model