Intest Res.
2021 Oct;19(4):461-467. 10.5217/ir.2020.00042.
Is there a correlation between infliximab trough levels and the development of adverse events in patients with inflammatory bowel disease?
- Affiliations
-
- 1Department of Gastroenterology, University Hospital of Heraklion, Medical School University of Crete, Heraklion, Greece
- 2Department of Basic Medical Sciences, Laboratory of Biology, National and Kapodistrian University of Athens Medical School, Athens, Greece
- KMID: 2521609
- DOI: http://doi.org/10.5217/ir.2020.00042
Abstract
- Background/Aims
The measurement of infliximab trough levels (IFX-TLs) in patients with inflammatory bowel disease (IBD) is performed to optimize treatment. However, the association between the development of adverse events (AEs) and IFX-TLs has not been sufficiently studied thus far. To investigate the possible association of IFX-TLs with AEs in Greek patients with IBD receiving maintenance treatment with IFX.
Methods
A retrospective analysis of the registry data of the Gastroenterology Department of the University Hospital of Heraklion, from IBD patients with at least one available IFX-TL measurement during the years 2016 to 2017 was conducted. AEs reported 4 months before and 4 months after the measured IFX-TLs were recorded. The IFX-TLs of patients with or without AEs were compared.
Results
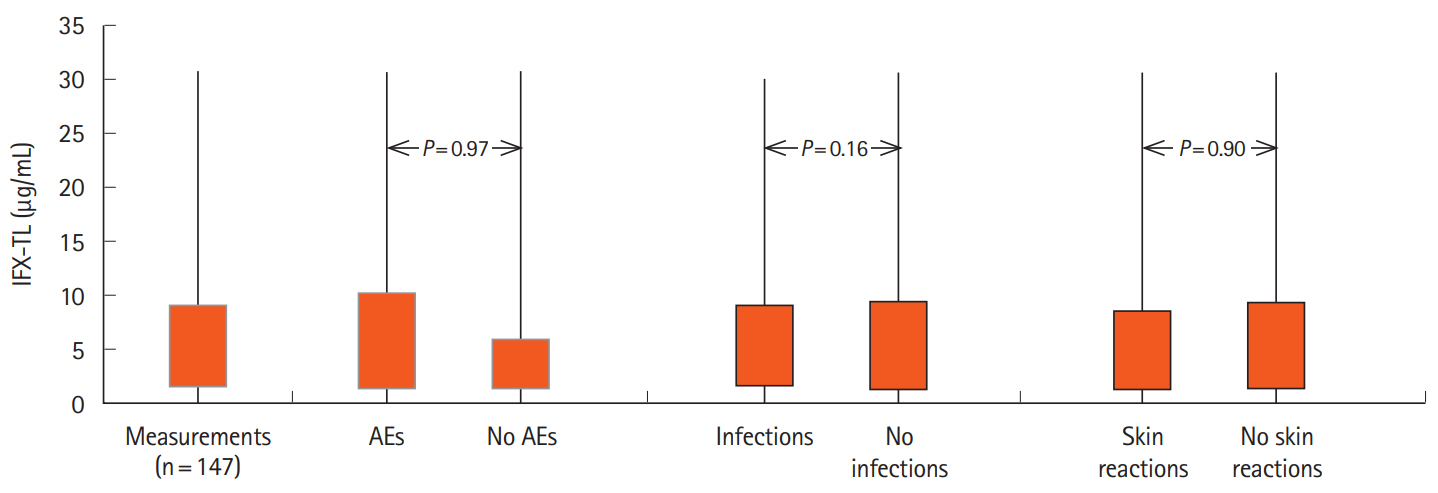
Of a total of 83 IBD patients (61 Crohn’s disease [73%]; 52 men [63%]; mean age ± standard deviation, 43.3 ± 16.0 years), 147 measurements of IFX-TLs were available (median 4.69 μg/ mL [1.32–9.16]), and 99 AEs (67.3%, 14 severe) were registered. The median IFX-TL of patients with AEs was 5.79 μg/mL (1.36– 10.25), higher than the median IFX-TL of patients without AEs (3.40 μg/mL [1.30–5.92]), but the difference was not significant (P= 0.97). The presence of infections or dermatologic reactions was not correlated with IFX-TLs. There was no difference in the prevalence of the total AEs (66.7% vs. 73.3%, P= 0.77) or in the analysis of AEs by group between patients with IFX-TLs ≥ 15 μg/ mL and patients with IFX-TLs < 15 μg/mL.
Conclusions
IFX-TLs are not significantly associated with the development of AEs in IBD patients receiving maintenance treatment with IFX.
Figure
Reference
-
1. Schnitzler F, Fidder H, Ferrante M, et al. Mucosal healing predicts long-term outcome of maintenance therapy with infliximab in Crohn’s disease. Inflamm Bowel Dis. 2009; 15:1295–1301.2. Colombel JF, Sandborn WJ, Rutgeerts P, et al. Comparison of two adalimumab treatment schedule strategies for moderate-to-severe Crohn’s disease: results from the CHARM trial. Am J Gastroenterol. 2009; 104:1170–1179.3. Papamichael K, Lin S, Moore M, Papaioannou G, Sattler L, Cheifetz AS. Infliximab in inflammatory bowel disease. Ther Adv Chronic Dis. 2019; 10:2040622319838443.4. van Hoeve K, Dreesen E, Hoffman I, et al. Higher infliximab trough levels are associated with better outcome in paediatric patients with inflammatory bowel disease. J Crohns Colitis. 2018; 12:1316–1325.5. Hoentjen F, van Bodegraven AA. Safety of anti-tumor necrosis factor therapy in inflammatory bowel disease. World J Gastroenterol. 2009; 15:2067–2073.6. Antoni C, Braun J. Side effects of anti-TNF therapy: current knowledge. Clin Exp Rheumatol. 2002; 20(6 Suppl 28):S152–S157.7. Lichtenstein L, Ron Y, Kivity S, et al. Infliximab-related infusion reactions: systematic review. J Crohns Colitis. 2015; 9:806–815.8. Kamperidis N, Middleton P, Tyrrell T, Stasinos I, Arebi N. Impact of therapeutic drug level monitoring on outcomes of patients with Crohn’s disease treated with infliximab: real world data from a retrospective single centre cohort study. Frontline Gastroenterol. 2019; 10:330–336.9. Quezada SM, McLean LP, Cross RK. Adverse events in IBD therapy: the 2018 update. Expert Rev Gastroenterol Hepatol. 2018; 12:1183–1191.10. Greener T, Kabakchiev B, Steinhart AH, Silverberg MS. Higher infliximab levels are not associated with an increase in adverse events in inflammatory bowel disease. Inflamm Bowel Dis. 2018; 24:1808–1814.11. Guiotto C, Daperno M, Frigerio F, et al. Clinical relevance and inter-test reliability of anti-infliximab antibodies and infliximab trough levels in patients with inflammatory bowel disease. Dig Liver Dis. 2016; 48:138–143.12. Huang V, Dhami N, Fedorak D, et al. A study investigating the association of dermatological and infusion reactions to infliximab and infliximab trough levels. Can J Gastroenterol Hepatol. 2015; 29:35–40.13. Protic M, Münger C, Seibold F. SA1267 psoriasis induced by anti TNF treatment in patients with inflammatory bowel disease: a single center study. Gastroenterology. 2014; 146(5 Supple 1):S247–S248.14. Cleynen I, Van Moerkercke W, Billiet T, et al. Anti‐TNF‐induced skin manifestations in IBD patients: role for increased drug exposure? Gastroenterology. 2015; 148(4 Suppl 1):S–108.15. Drobne D, Kurent T, Golob S, et al. Success and safety of high infliximab trough levels in inflammatory bowel disease. Scand J Gastroenterol. 2018; 53:940–946.16. Coutzac C, Chapuis J, Poullenot F, et al. Association between infliximab trough levels and the occurrence of paradoxical manifestations in patients with inflammatory bowel disease: a case-control study. J Crohns Colitis. 2015; 9:982–987.17. Moore C, Corbett G, Moss AC. Systematic review and meta-analysis: serum infliximab levels during maintenance therapy and outcomes in inflammatory bowel disease. J Crohns Colitis. 2016; 10:619–625.18. Orfanoudaki E, Gazouli M, Foteinogiannopoulou K, et al. Infliximab trough levels are decreasing over time in patients with inflammatory bowel disease on maintenance treatment with infliximab. Eur J Gastroenterol Hepatol. 2019; 31:187–191.19. Vermeire S, Noman M, Van Assche G, Baert F, D’Haens G, Rutgeerts P. Effectiveness of concomitant immunosuppressive therapy in suppressing the formation of antibodies to infliximab in Crohn’s disease. Gut. 2007; 56:1226–1231.20. Ben-Horin S, Waterman M, Kopylov U, et al. Addition of an immunomodulator to infliximab therapy eliminates antidrug antibodies in serum and restores clinical response of patients with inflammatory bowel disease. Clin Gastroenterol Hepatol. 2013; 11:444–447.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Early Infliximab Trough Levels Predict the Long-term Efficacy of Infliximab in a Randomized Controlled Trial in Patients with Active Crohn’s Disease Comparing, between CT-P13 and Originator Infliximab
- Serum Infliximab Cutoff trough Level Values for Maintaining Hematological Remission in Pediatric Inflammatory Bowel Disease
- Clinical Use of Measuring Trough Levels and Antibodies against Infliximab in Patients with Pediatric Inflammatory Bowel Disease
- Optic Neuritis after Infliximab Treatment in a Patient with Ulcerative Colitis
- Rectal tuberculosis after infliximab therapy despite negative screening for latent tuberculosis in a patient with ulcerative colitis