Intest Res.
2021 Oct;19(4):438-447. 10.5217/ir.2020.00055.
High mucosal cytomegalovirus DNA helps predict adverse short-term outcome in acute severe ulcerative colitis
- Affiliations
-
- 1Department of Gastroenterology, All India Institute of Medical Sciences, New Delhi, India
- 2Department of Microbiology, All India Institute of Medical Sciences, New Delhi, India
- 3Department of Gastrointestinal Surgery, All India Institute of Medical Sciences, New Delhi, India
- 4Department of Pathology, All India Institute of Medical Sciences, New Delhi, India
- 5Department of Radiodiagnosis, All India Institute of Medical Sciences, New Delhi, India
- 6Translational Gastroenterology Unit, NIHR Oxford Biomedical Research Centre, Oxford University Hospitals NHS Foundation Trust, John Radcliffe Hospital, Oxford, UK
- KMID: 2521607
- DOI: http://doi.org/10.5217/ir.2020.00055
Abstract
- Background/Aims
Predictors of short-term outcome of intravenous (IV) steroid therapy in acute severe ulcerative colitis (ASUC) have been well described, but the impact of cytomegalovirus (CMV) infection as a predictor of outcome remains debatable. We investigated the role of quantitative CMV polymerase chain reaction (PCR) as a predictor of short-term outcome in patients with ASUC.
Methods
Consecutive patients with ASUC satisfying Truelove and Witts criteria hospitalized at All India Institute of Medical Sciences (AIIMS) from May 2016 to July 2019 were included; all received IV steroid. The primary outcome measure was steroid-failure defined as the need for rescue therapy (with ciclosporin or infliximab) or colectomy during admission. AIIMS’ index (ulcerative colitis index of severity > 6 at day 1+fecal calprotectin > 1,000 μg/g at day 3), with quantitative CMV PCR on biopsy samples obtained at initial sigmoidoscopy were correlated with the primary outcome.
Results
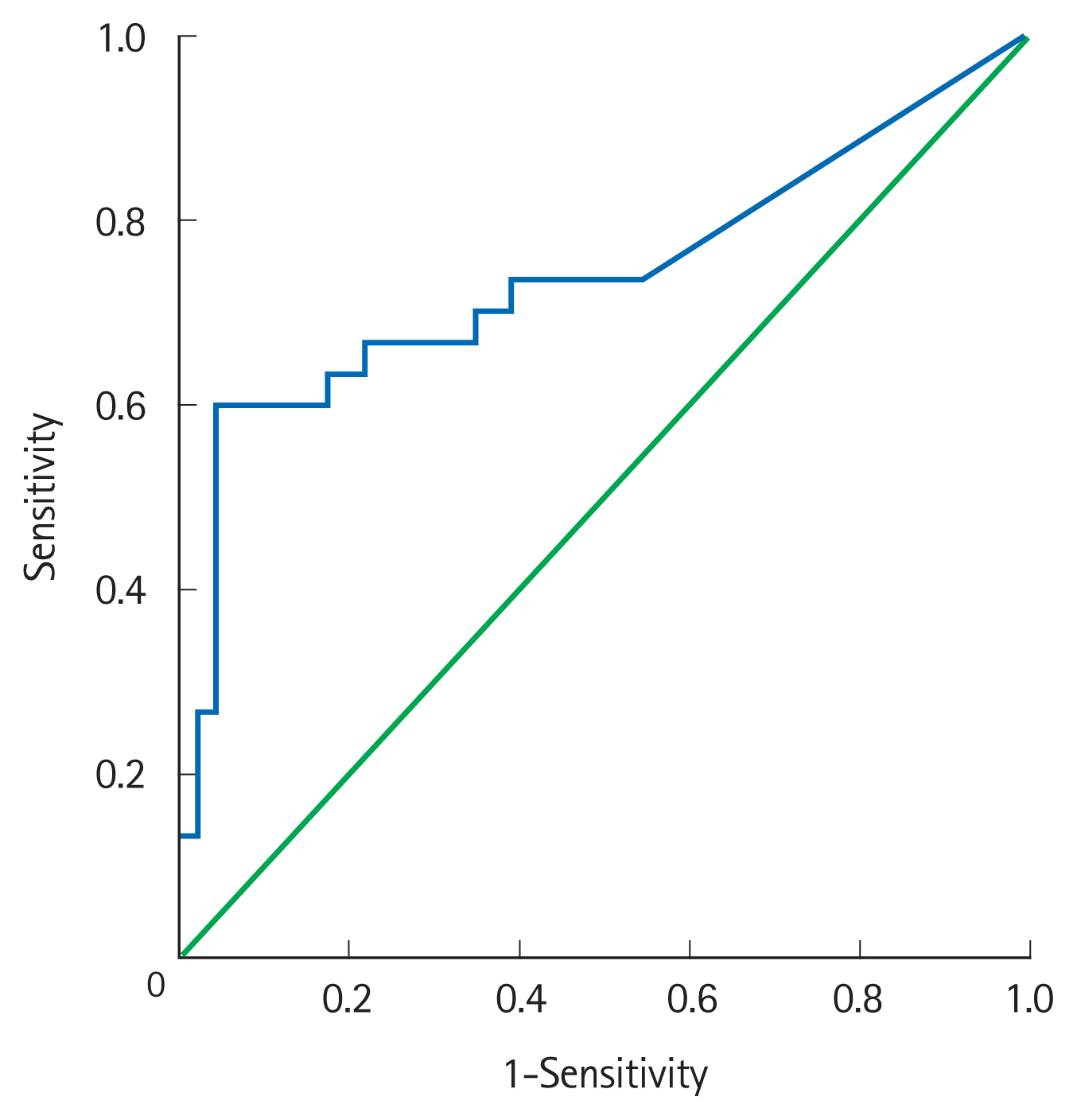
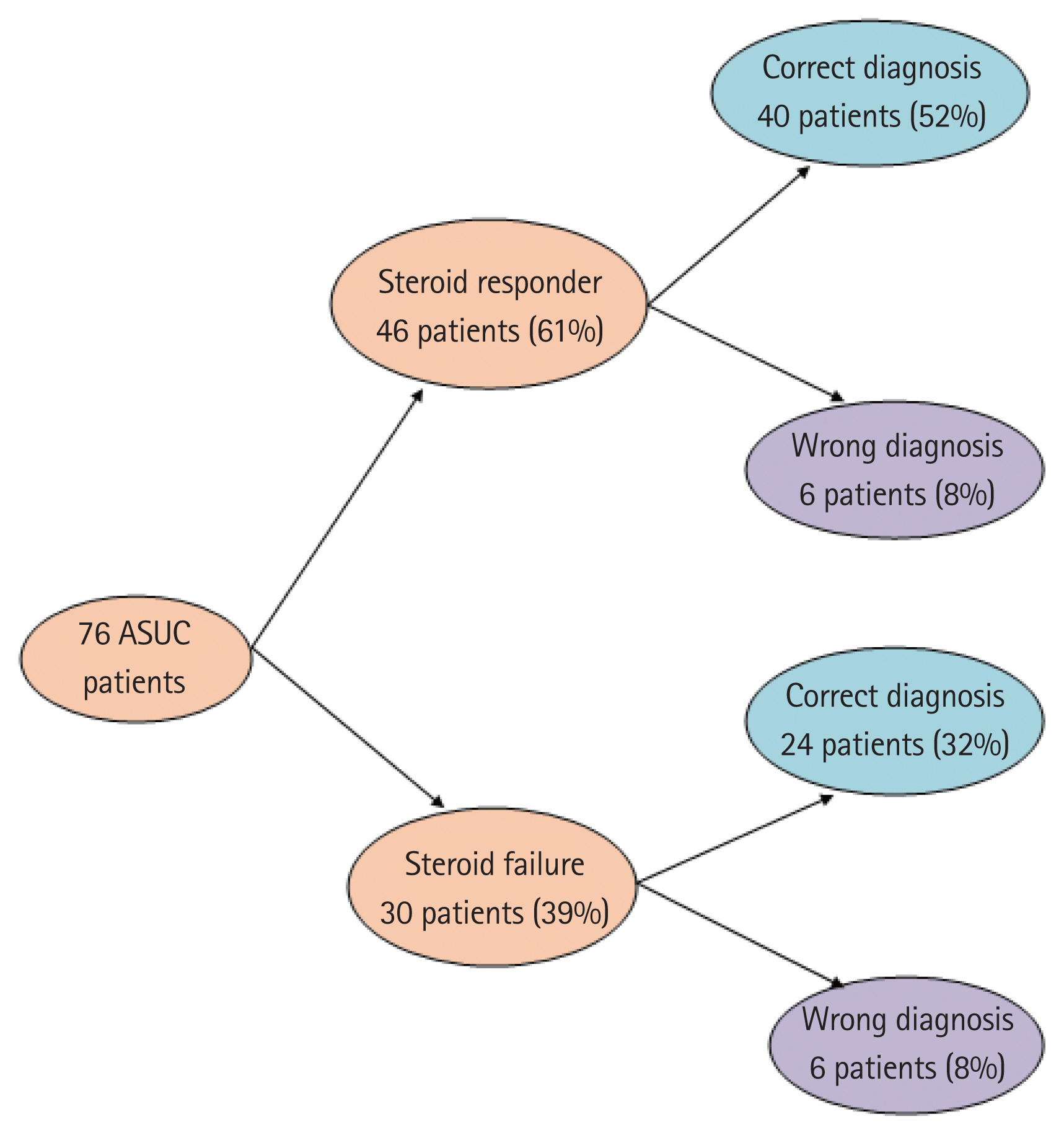
Thirty of 76 patients (39%) failed IV corticosteroids and 12 (16%) underwent surgery. Patients with steroid failure had a significantly higher mucosal CMV DNA than responders (3,454 copies/mg [0–2,700,000] vs. 116 copies/mg [0–27,220]; P< 0.01). On multivariable analysis, mucosal CMV DNA load > 2,000 copies/mg (odds ratio [OR], 10.2; 95% confidence interval [CI], 2.6–39.7; P< 0.01) and AIIMS’ index (OR, 39.8; 95% CI, 4.4–364.4; P< 0.01) were independent predictors of steroid-failure and need for colectomy. The combination correctly predicted outcomes in 84% of patients with ASUC.
Conclusions
High mucosal CMV DNA ( > 2,000 copies/mg) independently predicts failure of IV corticosteroids and short-term risk of colectomy and it has an additional value to the established markers of disease severity in patients with ASUC.
Figure
Reference
-
1. Dinesen LC, Walsh AJ, Protic MN, et al. The pattern and outcome of acute severe colitis. J Crohns Colitis. 2010; 4:431–437.
Article2. Cesarini M, Collins GS, Rönnblom A, et al. Predicting the individual risk of acute severe colitis at diagnosis. J Crohns Colitis. 2017; 11:335–341.
Article3. Turner D, Walsh CM, Steinhart AH, Griffiths AM. Response to corticosteroids in severe ulcerative colitis: a systematic review of the literature and a meta-regression. Clin Gastroenterol Hepatol. 2007; 5:103–110.4. Travis SP, Farrant JM, Ricketts C, et al. Predicting outcome in severe ulcerative colitis. Gut. 1996; 38:905–910.5. Lindgren SC, Flood LM, Kilander AF, Löfberg R, Persson TB, Sjödahl RI. Early predictors of glucocorticosteroid treatment failure in severe and moderately severe attacks of ulcerative colitis. Eur J Gastroenterol Hepatol. 1998; 10:831–835.
Article6. Jain S, Kedia S, Bopanna S, et al. Faecal calprotectin and UCEIS predict short-term outcomes in acute severe colitis: prospective cohort study. J Crohns Colitis. 2017; 11:1309–1316.7. Le Baut G, Kirchgesner J, Amiot A, et al. A scoring system to determine patients’ risk of colectomy within 1 year after hospital admission for acute severe ulcerative colitis. Clin Gastroenterol Hepatol. 2021; 19:1602–1610.8. Papadakis KA, Tung JK, Binder SW, et al. Outcome of cytomegalovirus infections in patients with inflammatory bowel disease. Am J Gastroenterol. 2001; 96:2137–2142.9. Lee HS, Park SH, Kim SH, et al. Risk factors and clinical outcomes associated with cytomegalovirus colitis in patients with acute severe ulcerative colitis. Inflamm Bowel Dis. 2016; 22:912–918.10. Grossberg LB, Ezaz G, Grunwald D, Cohen J, Falchuk KR, Feuerstein JD. A national survey of the prevalence and impact of cytomegalovirus infection among hospitalized patients with ulcerative colitis. J Clin Gastroenterol. 2018; 52:241–245.11. Zagórowicz E, Bugajski M, Wieszczy P, Pietrzak A, Magdziak A, Mróz A. Cytomegalovirus infection in ulcerative colitis is related to severe inflammation and a high count of cytomegalovirus-positive cells in biopsy is a risk factor for colectomy. J Crohns Colitis. 2016; 10:1205–1211.12. Levin A, Yaari S, Stoff R, Caplan O, Wolf DG, Israeli E. Diagnosis of cytomegalovirus infection during exacerbation of ulcerative colitis. Digestion. 2017; 96:142–148.13. Yoshino T, Nakase H, Ueno S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007; 13:1516–1521.14. Okahara K, Nagata N, Shimada T, et al. Colonic cytomegalovirus detection by mucosal PCR and antiviral therapy in ulcerative colitis. PLoS One. 2017; 12:e0183951.15. Roblin X, Pillet S, Oussalah A, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011; 106:2001–2008.
Article16. Dignass A, Eliakim R, Magro F, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 1: definitions and diagnosis. J Crohns Colitis. 2012; 6:965–990.17. Truelove SC, Witts LJ. Cortisone in ulcerative colitis; preliminary report on a therapeutic trial. Br Med J. 1954; 2:375–378.18. Magro F, Gionchetti P, Eliakim R, et al. Third European evidence-based consensus on diagnosis and management of ulcerative colitis. part 1: definitions, diagnosis, extra-intestinal manifestations, pregnancy, cancer surveillance, surgery, and ileo-anal pouch disorders. J Crohns Colitis. 2017; 11:649–670.19. Silverberg MS, Satsangi J, Ahmad T, et al. Toward an integrated clinical, molecular and serological classification of inflammatory bowel disease: report of a Working Party of the 2005 Montreal World Congress of Gastroenterology. Can J Gastroenterol. 2005; 19(Suppl A):5A–36A.20. Travis SP, Schnell D, Krzeski P, et al. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013; 145:987–995.21. Bradford RD, Cloud G, Lakeman AD, et al. Detection of cytomegalovirus (CMV) DNA by polymerase chain reaction is associated with hearing loss in newborns with symptomatic congenital CMV infection involving the central nervous system. J Infect Dis. 2005; 191:227–233.22. Griscelli F, Barrois M, Chauvin S, Lastere S, Bellet D, Bourhis JH. Quantification of human cytomegalovirus DNA in bone marrow transplant recipients by real-time PCR. J Clin Microbiol. 2001; 39:4362–4369.23. Banerjee D, Deb R, Dar L, et al. High frequency of parasitic and viral stool pathogens in patients with active ulcerative colitis: report from a tropical country. Scand J Gastroenterol. 2009; 44:325–331.24. Nakase H, Yoshino T, Ueno S, et al. Importance of early detection of cytomegalovirus infection in refractory inflammatory bowel disease. Inflamm Bowel Dis. 2007; 13:364.
Article25. Clos-Parals A, Rodríguez-Martínez P, Cañete F, et al. Prognostic value of the burden of cytomegalovirus colonic reactivation evaluated by immunohistochemical staining in patients with active ulcerative colitis. J Crohns Colitis. 2019; 13:385–388.26. Landry ML, Ferguson D. Comparison of quantitative cytomegalovirus antigenemia assay with culture methods and correlation with clinical disease. J Clin Microbiol. 1993; 31:2851–2856.27. Kou T, Nakase H, Tamaki H, Kudo T, Nishio A, Chiba T. Cytomegalovirus infection in patients with ulcerative colitis diagnosed by quantitative real-time PCR analysis. Dig Dis Sci. 2006; 51:1052–1055.
Article28. Cohen S, Martinez-Vinson C, Aloi M, et al. Cytomegalovirus infection in pediatric severe ulcerative colitis-a multicenter study from the pediatric inflammatory bowel disease Porto group of the European society of pediatric gastroenterology, hepatology and nutrition. Pediatr Infect Dis J. 2018; 37:197–201.29. Criscuoli V, Rizzuto MR, Gallo E, Orlando A, Cottone M. Toxic megacolon and human cytomegalovirus in a series of severe ulcerative colitis patients. J Clin Virol. 2015; 66:103–106.30. Chun J, Lee C, Kwon JE, et al. Usefulness of the cytomegalovirus antigenemia assay in patients with ulcerative colitis. Intest Res. 2015; 13:50–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Case of Cytomegalvirus Colitis Developed during the Treatment of Ulcerative Colitis
- Does cytomegalovirus load predict the outcome of acute severe ulcerative colitis?
- Cytomegalovirus Colitis with Ulcerative Colitis in the Steroid Naive Immunocompetent Patient
- Approach to cytomegalovirus infections in patients with ulcerative colitis
- A Clinical Significance of Assessing Cytomegalovirus Infection Status in Patients With Ulcerative Colitis