Cancer Res Treat.
2021 Oct;53(4):1195-1203. 10.4143/crt.2020.1337.
Real-World Clinical Outcomes and Prognostic Factors for Patients with Advanced Angiosarcoma who Received Systemic Treatment
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul, Korea
- 2Cancer Research Institute, Seoul National University, Seoul, Korea
- 3Department of Pathology, Seoul National University Hospital, Seoul, Korea
- 4Department of Internal Medicine, Seoul National University Bundang Hospital, Seongnam, Korea
- KMID: 2521594
- DOI: http://doi.org/10.4143/crt.2020.1337
Abstract
- Purpose
Angiosarcoma is a highly aggressive mesenchymal tumor. Although systemic chemotherapy is often considered for the inoperable or metastatic angiosarcoma, the outcome of such treatment is unsatisfactory and poorly delineated.
Materials and Methods
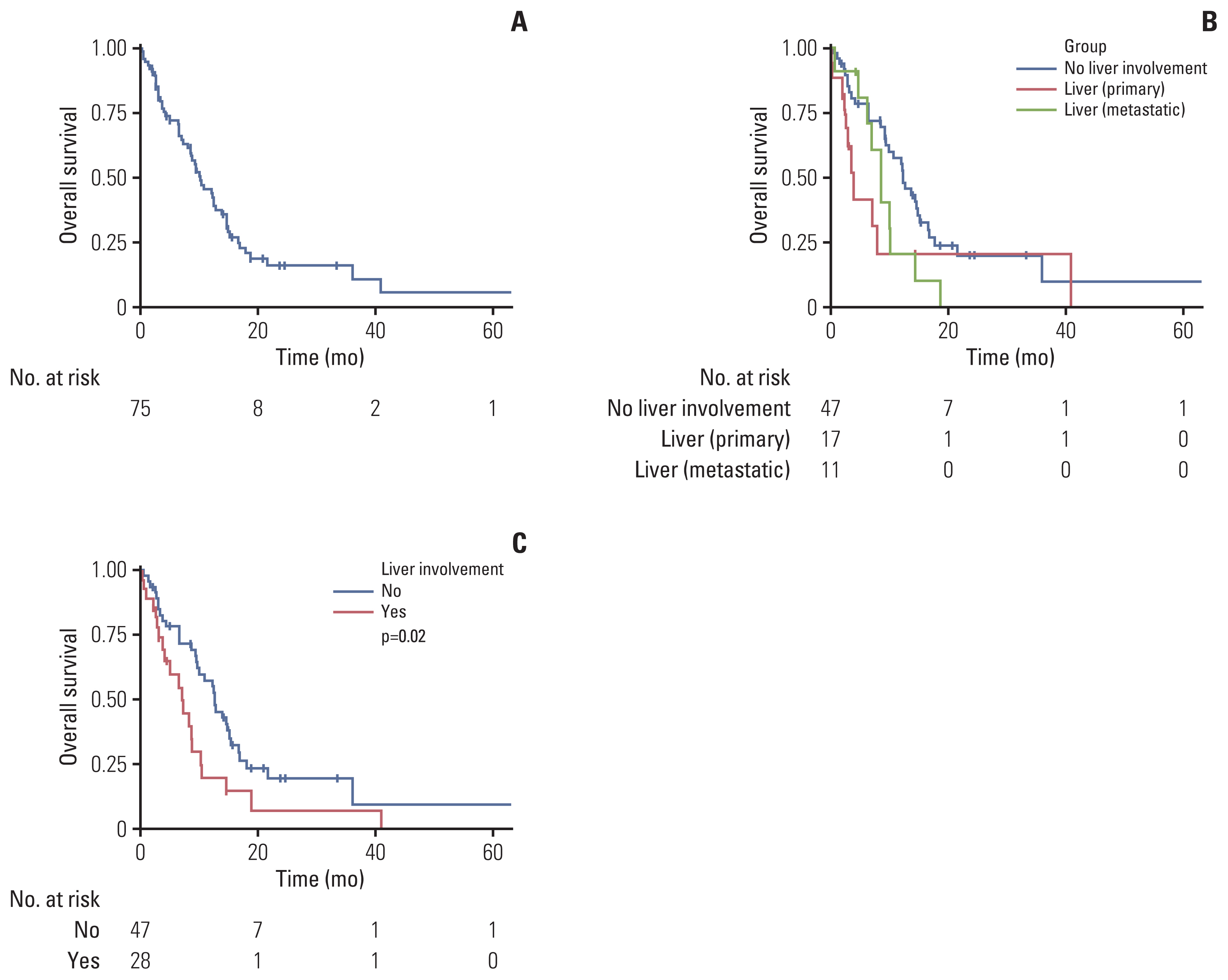
We reviewed electronic medical records of 75 patients with angiosarcoma who were treated with systemic chemotherapy for inoperable or metastatic disease. Patients were classified as having liver involvement if they had either primary or metastatic hepatic lesions.
Results
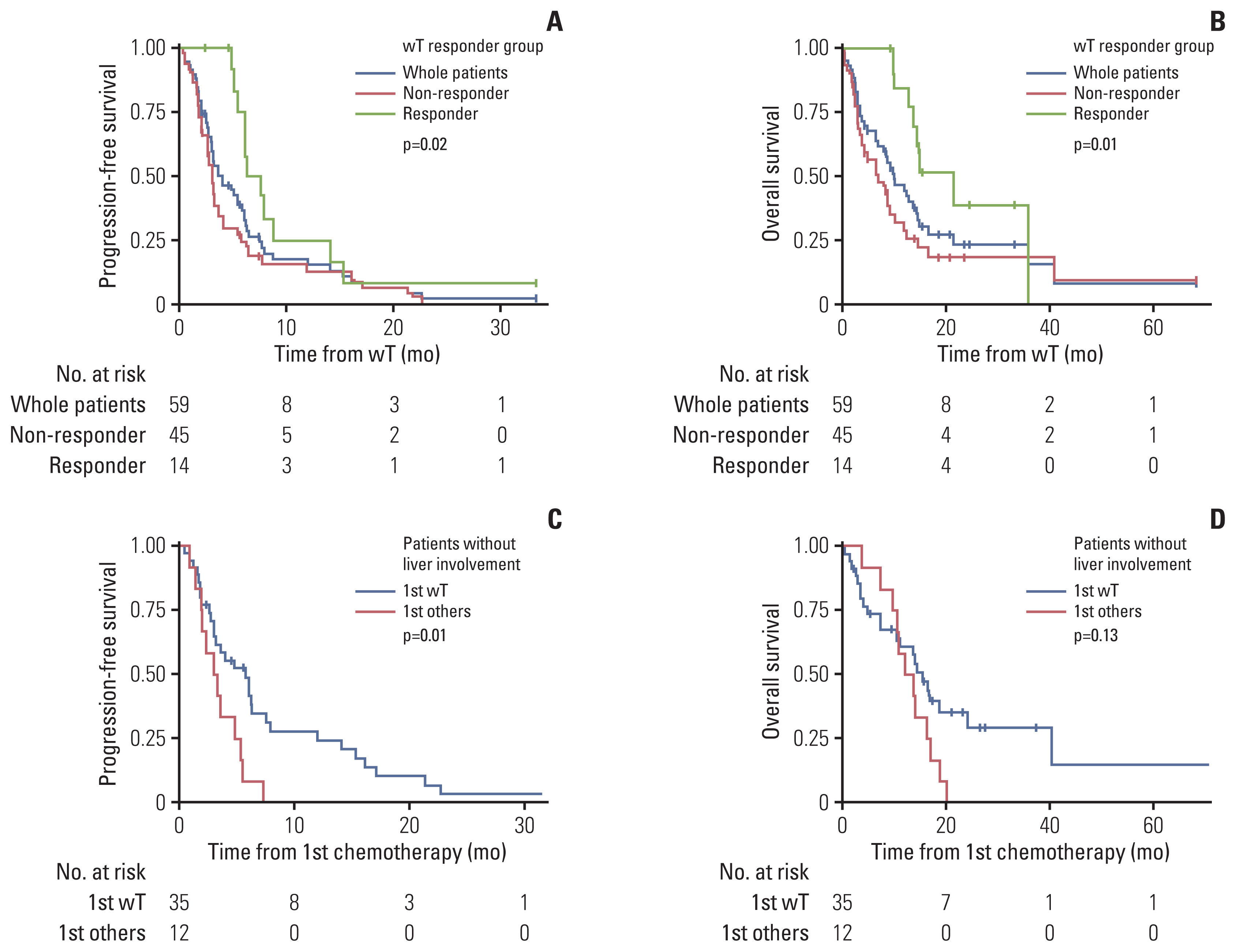
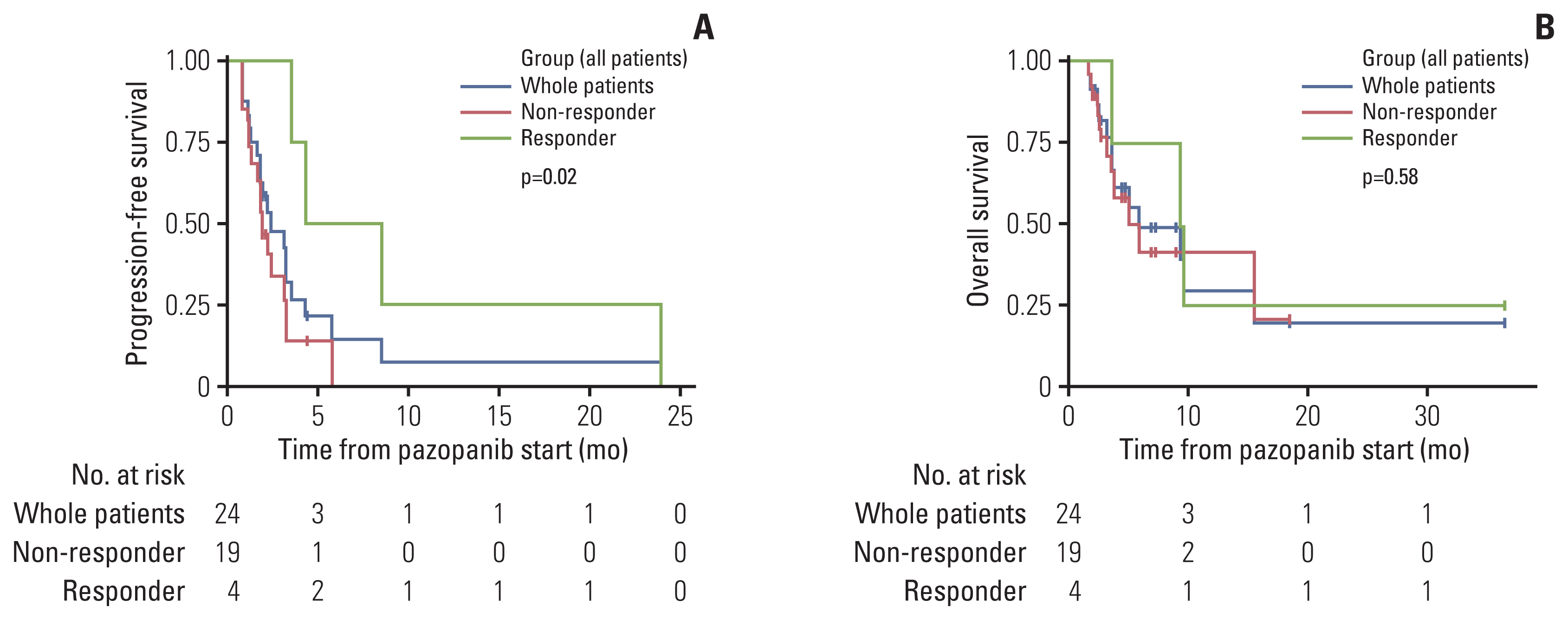
Among the patients evaluated, 51 patients were male (68%) and 24 patients (32%) had primary cutaneous angiosarcoma. Liver involvement was present in 28 patients (37.3%). A total of 59 patients received first-line weekly paclitaxel (wPac) and showed an objective response rate (ORR) of 23.7% (n=14), a median progression free survival (mPFS) of 4.0 months (95% confidence interval [CI] 3.0–6.1), and a median overall survival (mOS) of 10.2 months (95% CI 7.0–14.6). Among patients without liver involvement, patients receiving wPac (n=35) had significantly prolonged mPFS (5.8 vs. 3.2 months, respectively, p=0.014) with a tendency for prolonged mOS (13.8 vs. 11.6 months, respectively, p=0.13) than those receiving other regimens (n=12). A total of 24 patients received second- or later-line pazopanib monotherapy and showed an ORR of 16.7% (n=4), a mPFS of 2.4 months (95% CI 1.8–4.3) and a mOS of 5.4 months (95% CI 3.5–NA).
Conclusion
Treatment with first-line wPac and later-line pazopanib seems to provide survival benefit, especially for patients with advanced angiosarcoma without liver involvement.
Keyword
Figure
Reference
-
References
1. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol. 2010; 11:983–91.
Article2. Lahat G, Dhuka AR, Hallevi H, Xiao L, Zou C, Smith KD, et al. Angiosarcoma: clinical and molecular insights. Ann Surg. 2010; 251:1098–106.3. Abraham JA, Hornicek FJ, Kaufman AM, Harmon DC, Springfield DS, Raskin KA, et al. Treatment and outcome of 82 patients with angiosarcoma. Ann Surg Oncol. 2007; 14:1953–67.
Article4. Fayette J, Martin E, Piperno-Neumann S, Le Cesne A, Robert C, Bonvalot S, et al. Angiosarcomas, a heterogeneous group of sarcomas with specific behavior depending on primary site: a retrospective study of 161 cases. Ann Oncol. 2007; 18:2030–6.
Article5. Penel N, Marreaud S, Robin YM, Hohenberger P. Angiosarcoma: state of the art and perspectives. Crit Rev Oncol Hematol. 2011; 80:257–63.
Article6. Young RJ, Natukunda A, Litiere S, Woll PJ, Wardelmann E, van der Graaf WT. First-line anthracycline-based chemotherapy for angiosarcoma and other soft tissue sarcoma subtypes: pooled analysis of eleven European Organisation for Research and Treatment of Cancer Soft Tissue and Bone Sarcoma Group trials. Eur J Cancer. 2014; 50:3178–86.
Article7. Penel N, Bui BN, Bay JO, Cupissol D, Ray-Coquard I, Piperno-Neumann S, et al. Phase II trial of weekly paclitaxel for unresectable angiosarcoma: the ANGIOTAX Study. J Clin Oncol. 2008; 26:5269–74.
Article8. Weidema ME, Versleijen-Jonkers YM, Flucke UE, Desar IM, van der Graaf WT. Targeting angiosarcomas of the soft tissues: a challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol. 2019; 138:120–31.
Article9. Tokuyama W, Mikami T, Masuzawa M, Okayasu I. Autocrine and paracrine roles of VEGF/VEGFR-2 and VEGF-C/VEGFR-3 signaling in angiosarcomas of the scalp and face. Hum Pathol. 2010; 41:407–14.
Article10. Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol. 2008; 97:74–81.
Article11. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol. 2013; 24:257–63.
Article12. van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012; 379:1879–86.
Article13. Fletcher CD, Brdige JA, Hogendoorn PC, Mertens F. WHO classification of tumours of soft tissue and bone. 4th ed. Lyon: IARC Press;2013.14. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45:228–47.
Article15. Common terminology criteria for adverse events, v5.0 [Internet]. Waltham, MA: UpToDate;2017. [cited 2018 May 1]. Available from: http://www.uptodate.com/contents/common-terminology-criteria-for-adverse-events .16. Wang L, Lao IW, Yu L, Wang J. Clinicopathological features and prognostic factors in angiosarcoma: A retrospective analysis of 200 patients from a single Chinese medical institute. Oncol Lett. 2017; 14:5370–8.
Article17. Penel N, Italiano A, Ray-Coquard I, Chaigneau L, Delcambre C, Robin YM, et al. Metastatic angiosarcomas: doxorubicin-based regimens, weekly paclitaxel and metastasectomy significantly improve the outcome. Ann Oncol. 2012; 23:517–23.
Article18. Ray-Coquard IL, Domont J, Tresch-Bruneel E, Bompas E, Cassier PA, Mir O, et al. Paclitaxel given once per week with or without bevacizumab in patients with advanced angiosarcoma: a randomized phase II trial. J Clin Oncol. 2015; 33:2797–802.
Article19. Byeon S, Song HN, Kim HK, Ham JS, Lee SJ, Lee J, et al. A Korean single-center, real-world, retrospective study of first-line weekly paclitaxel in patients with metastatic angiosarcoma. Clin Sarcoma Res. 2016; 6:8.
Article20. Kollar A, Jones RL, Stacchiotti S, Gelderblom H, Guida M, Grignani G, et al. Pazopanib in advanced vascular sarcomas: an EORTC Soft Tissue and Bone Sarcoma Group (STBSG) retrospective analysis. Acta Oncol. 2017; 56:88–92.21. Zietz C, Rossle M, Haas C, Sendelhofert A, Hirschmann A, Sturzl M, et al. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol. 1998; 153:1425–33.
Article22. Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet. 2014; 46:376–9.
Article23. Bocci G, Di Paolo A, Danesi R. The pharmacological bases of the antiangiogenic activity of paclitaxel. Angiogenesis. 2013; 16:481–92.
Article24. Schutz FA, Choueiri TK, Sternberg CN. Pazopanib: Clinical development of a potent anti-angiogenic drug. Crit Rev Oncol Hematol. 2011; 77:163–71.
Article25. Wagner MJ, Ravi V, Menter DG, Sood AK. Endothelial cell malignancies: new insights from the laboratory and clinic. NPJ Precis Oncol. 2017; 1:11.
Article26. Lee SW, Song CY, Gi YH, Kang SB, Kim YS, Nam SW, et al. Hepatic angiosarcoma manifested as recurrent hemoperitoneum. World J Gastroenterol. 2008; 14:2935–8.
Article27. Bhati CS, Bhatt AN, Starkey G, Hubscher SG, Bramhall SR. Acute liver failure due to primary angiosarcoma: a case report and review of literature. World J Surg Oncol. 2008; 6:104.
Article28. Chang YP, Chen YM, Lai CH, Lin CY, Fang WF, Huang CH, et al. The impact of de novo liver metastasis on clinical outcome in patients with advanced non-small-cell lung cancer. PLoS One. 2017; 12:e0178676.
Article29. Tumeh PC, Hellmann MD, Hamid O, Tsai KK, Loo KL, Gubens MA, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res. 2017; 5:417–24.
Article30. Frentzas S, Simoneau E, Bridgeman VL, Vermeulen PB, Foo S, Kostaras E, et al. Vessel co-option mediates resistance to anti-angiogenic therapy in liver metastases. Nat Med. 2016; 22:1294–302.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Analysis of Factors Predicting the Real-World Efficacy of Atezolizumab and Bevacizumab in Patients with Advanced Hepatocellular Carcinoma
- Real-World Treatment Patterns and Clinical Outcomes in Korean Patients With AML Ineligible for First-Line Intensive Chemotherapy: A Subanalysis of the CURRENT Study, a Non-Interventional, Retrospective Chart Review
- Treatment Outcome of Scalp Angiosarcoma: A Single Institute Experience
- First-Line Alectinib vs. Brigatinib in Advanced Non–Small Cell Lung Cancer with ALK Rearrangement: Real-World Data
- Long-term efficacy and safety of tofacitinib in patients with ulcerative colitis: 3-year results from a real-world study