Cancer Res Treat.
2021 Oct;53(4):1174-1183. 10.4143/crt.2021.031.
Ferroportin and FBXL5 as Prognostic Markers in Advanced Stage Clear Cell Renal Cell Carcinoma
- Affiliations
-
- 1Department of Pathology, Yonsei University College of Medicine, Seoul, Korea
- 2Division of Hemato-Oncology, National Health Insurance Service (NHIS) Ilsan Hospital, Goyang, Korea
- 3Department of Urology and Urological Science Institute, Yonsei University College of Medicine, Seoul, Korea
- 4Division of Medical Oncology, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2521592
- DOI: http://doi.org/10.4143/crt.2021.031
Abstract
- Purpose
Advanced stage clear cell renal cell carcinoma (ccRCC) involves a poor prognosis. Several studies have reported that dysfunctions in iron metabolism‒related proteins may cause tumor progression and metastasis of this carcinoma. In this study, we investigated the impact of the expression of iron metabolism‒related proteins on patient prognoses in advanced stage ccRCCs.
Materials and Methods
All of 143 advanced stage ccRCC specimens were selected following validation with double blind reviews. Several clinicopathological parameters including nuclear grade, perirenal fat invasion, renal sinus fat invasion, vascular invasion, necrosis, and sarcomatoid/rhabdoid differentiation were compared with the expression of ferroportin (FPN), and F-Box and leucine rich repeat protein 5 (FBXL5), by immunohistochemistry. FPN and FBXL5 mRNA level of ccRCC from The Cancer Genome Atlas database were also analyzed for validation.
Results
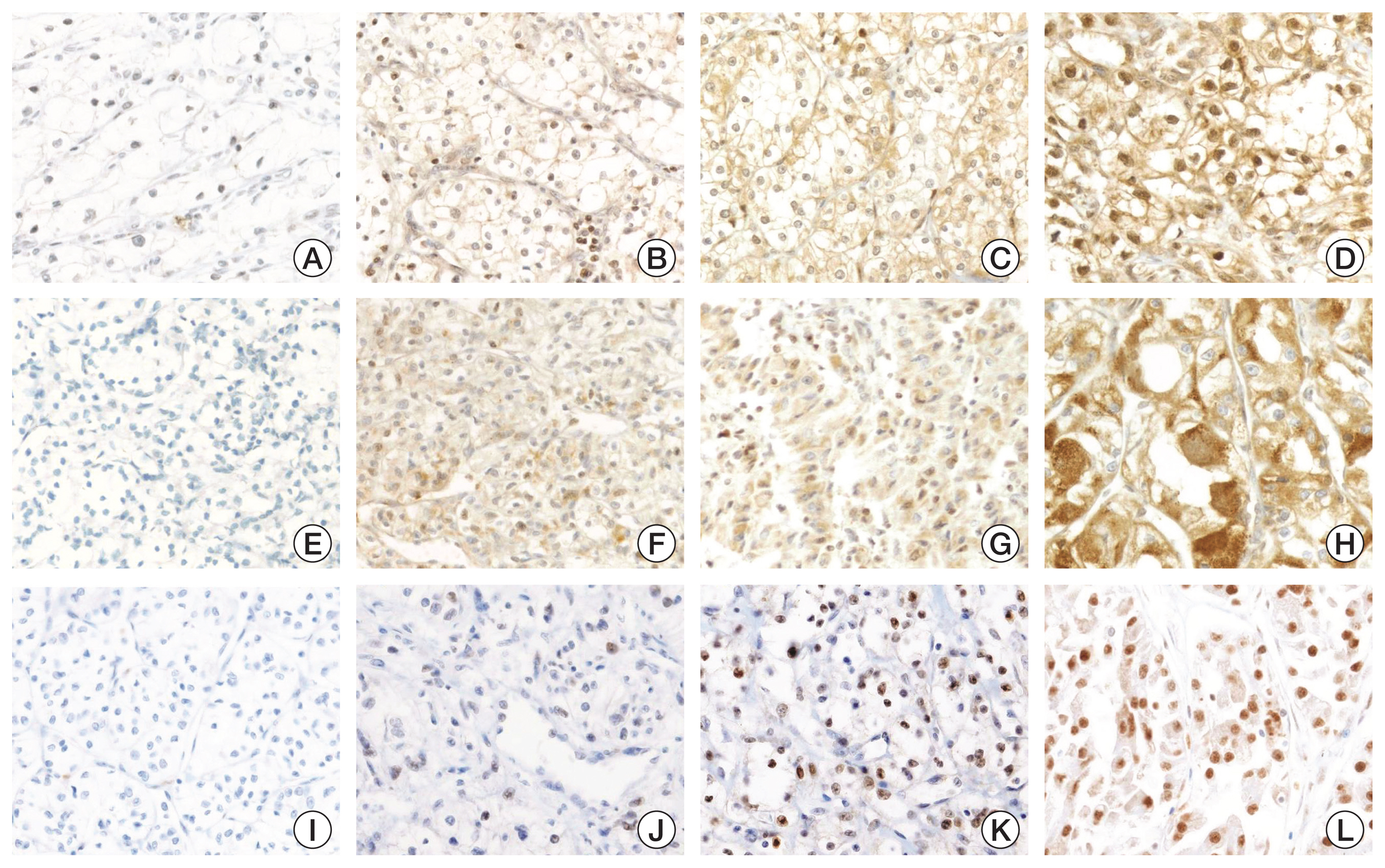
FPN and FBXL5 immunohistochemistry showed membrane and cytoplasmic expression, respectively. Based on the H-score, cases were classified as low or high expression with a cutoff value of 20 for FPN and 15 for FBXL5, respectively. Low expression of FPN and FBXL5 were significantly associated with patient death (p=0.022 and p=0.005, respectively). In survival analyses, low expression of FPN and FBXL5 were significantly associated with shorter overall survival (p=0.003 and p=0.004, respectively). On multivariate analysis, low expression of FBXL5 (hazard ratio, 2.001; p=0.034) was significantly associated with shorter overall survival.
Conclusion
FPN and FBXL5 can be used as potential prognostic markers and therapeutic targets for advanced stage ccRCC.
Keyword
Figure
Reference
-
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018; 68:394–424.
Article2. Linehan WM, Ricketts CJ. The Cancer Genome Atlas of renal cell carcinoma: findings and clinical implications. Nat Rev Urol. 2019; 16:539–52.
Article3. Linehan WM, Schmidt LS, Crooks DR, Wei D, Srinivasan R, Lang M, et al. The metabolic basis of kidney cancer. Cancer Discov. 2019; 9:1006–21.
Article4. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours. Eur Urol. 2016; 70:93–105.
Article5. Shen C, Kaelin WG Jr. The VHL/HIF axis in clear cell renal carcinoma. Semin Cancer Biol. 2013; 23:18–25.
Article6. Qian CN, Huang D, Wondergem B, Teh BT. Complexity of tumor vasculature in clear cell renal cell carcinoma. Cancer. 2009; 115:2282–9.
Article7. Atkins MB, Tannir NM. Current and emerging therapies for first-line treatment of metastatic clear cell renal cell carcinoma. Cancer Treat Rev. 2018; 70:127–37.
Article8. Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007; 356:115–24.
Article9. Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010; 28:1061–8.
Article10. Wang Y, Yu L, Ding J, Chen Y. Iron metabolism in cancer. Int J Mol Sci. 2018; 20:95.
Article11. Guo W, Zhang S, Chen Y, Zhang D, Yuan L, Cong H, et al. An important role of the hepcidin-ferroportin signaling in affecting tumor growth and metastasis. Acta Biochim Biophys Sin (Shanghai). 2015; 47:703–15.
Article12. Muto Y, Moroishi T, Ichihara K, Nishiyama M, Shimizu H, Eguchi H, et al. Disruption of FBXL5-mediated cellular iron homeostasis promotes liver carcinogenesis. J Exp Med. 2019; 216:950–65.
Article13. Moroishi T, Nishiyama M, Takeda Y, Iwai K, Nakayama KI. The FBXL5-IRP2 axis is integral to control of iron metabolism in vivo. Cell Metab. 2011; 14:339–51.
Article14. Wang L, Liu X, You LH, Ci YZ, Chang S, Yu P, et al. Hepcidin and iron regulatory proteins coordinately regulate ferroportin 1 expression in the brain of mice. J Cell Physiol. 2019; 234:7600–7.
Article15. Robinson RL, Sharma A, Bai S, Heneidi S, Lee TJ, Kodeboyina SK, et al. Comparative STAT3-regulated gene expression profile in renal cell carcinoma subtypes. Front Oncol. 2019; 9:72.
Article16. Xue X, Ramakrishnan SK, Weisz K, Triner D, Xie L, Attili D, et al. Iron uptake via DMT1 integrates cell cycle with JAK-STAT3 signaling to promote colorectal tumorigenesis. Cell Metab. 2016; 24:447–61.
Article17. Mou Y, Zhang Y, Wu J, Hu B, Zhang C, Duan C, et al. The landscape of iron metabolism-related and methylated genes in the prognosis prediction of clear cell renal cell carcinoma. Front Oncol. 2020; 10:788.
Article18. Delahunt B, Eble JN, Egevad L, Samaratunga H. Grading of renal cell carcinoma. Histopathology. 2019; 74:4–17.
Article19. Park CK, Shin SJ, Cho YA, Joo JW, Cho NH. HoxB13 expression in ductal type adenocarcinoma of prostate: clinicopathologic characteristics and its utility as potential diagnostic marker. Sci Rep. 2019; 9:20205.
Article20. Cho YA, Chung JM, Ryu H, Kim EK, Cho BC, Yoon SO. Investigating Trk protein expression between oropharyngeal and non-oropharyngeal squamous cell carcinoma: clinical implications and possible roles of human papillomavirus infection. Cancer Res Treat. 2019; 51:1052–63.
Article21. Camp RL, Dolled-Filhart M, Rimm DL. X-tile: a new bio- informatics tool for biomarker assessment and outcome-based cut-point optimization. Clin Cancer Res. 2004; 10:7252–9.22. Wu YY, Jiang JN, Fang XD, Ji FJ. STEAP1 regulates tumorigenesis and chemoresistance during peritoneal metastasis of Gastric Cancer. Front Physiol. 2018; 9:1132.
Article23. Imlay JA, Chin SM, Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988; 240:640–2.
Article24. Sun H, Zhang C, Cao S, Sheng T, Dong N, Xu Y. Fenton reactions drive nucleotide and ATP syntheses in cancer. J Mol Cell Biol. 2018; 10:448–59.
Article25. Schodel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, et al. Hypoxia, hypoxia-inducible transcription factors, and renal cancer. Eur Urol. 2016; 69:646–57.26. Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003; 23:9361–74.27. Menezes SV, Sahni S, Kovacevic Z, Richardson DR. Interplay of the iron-regulated metastasis suppressor NDRG1 with epidermal growth factor receptor (EGFR) and oncogenic signaling. J Biol Chem. 2017; 292:12772–82.
Article28. Chen XL, Lei L, Hong LL, Ling ZQ. Potential role of NDRG2 in reprogramming cancer metabolism and epithelial-to-mesenchymal transition. Histol Histopathol. 2018; 33:655–63.29. Xue D, Zhou CX, Shi YB, Lu H, He XZ. Decreased expression of ferroportin in prostate cancer. Oncol Lett. 2015; 10:913–6.
Article30. Pinnix ZK, Miller LD, Wang W, D’Agostino R Jr, Kute T, Willingham MC, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med. 2010; 2:43ra56.
Article31. Toshiyama R, Konno M, Eguchi H, Asai A, Noda T, Koseki J, et al. Association of iron metabolic enzyme hepcidin expression levels with the prognosis of patients with pancreatic cancer. Oncol Lett. 2018; 15:8125–33.
Article32. Boult J, Roberts K, Brookes MJ, Hughes S, Bury JP, Cross SS, et al. Overexpression of cellular iron import proteins is associated with malignant progression of esophageal adenocarcinoma. Clin Cancer Res. 2008; 14:379–87.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Correlation of Clinical Stage and Presumptive Prognostic Factors in Renal Cell Carcinoma
- The Prognostic Implications of Cystic Change in Clear Cell Renal Cell Carcinoma
- Differentiation of Chromophobe Renal Cell Carcinoma and Clear Cell Renal Cell Carcinoma by Using Helical CT
- A Case of Metastatic Renal Cell Carcinoma to the Gallbladder
- The Prognostic Value of Fuhrman Nuclear Grade, 1997 TNM Classification and cell Type in Renal Cell Carcinoma