Cancer Res Treat.
2021 Oct;53(4):1096-1103. 10.4143/crt.2020.928.
Phase II Trial of Postoperative Adjuvant Gemcitabine and Cisplatin Chemotherapy Followed by Chemoradiotherapy with Gemcitabine in Patients with Resected Pancreatic Cancer
- Affiliations
-
- 1Department of Internal Medicine, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 2Cancer Research Institute, Seoul National University, Seoul, Korea
- 3Department of Radiation Oncology, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 4Institute of Radiation Medicine, Medical Research Center, Seoul National University, Seoul, Korea
- 5Department of Internal Medicine, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 6Department of Internal Medicine, Chungbuk National University College of Medicine, Cheongju, Korea
- 7Department of Surgery, Seoul National University Hospital, Seoul National University College of Medicine, Seoul, Korea
- 8Department of Radiation Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- KMID: 2521584
- DOI: http://doi.org/10.4143/crt.2020.928
Abstract
- Purpose
Despite curative resection, the 5-year survival for patients with resectable pancreatic cancer is less than 20%. Recurrence occurs both locally and at distant sites and effective multimodality adjuvant treatment is needed.
Materials and Methods
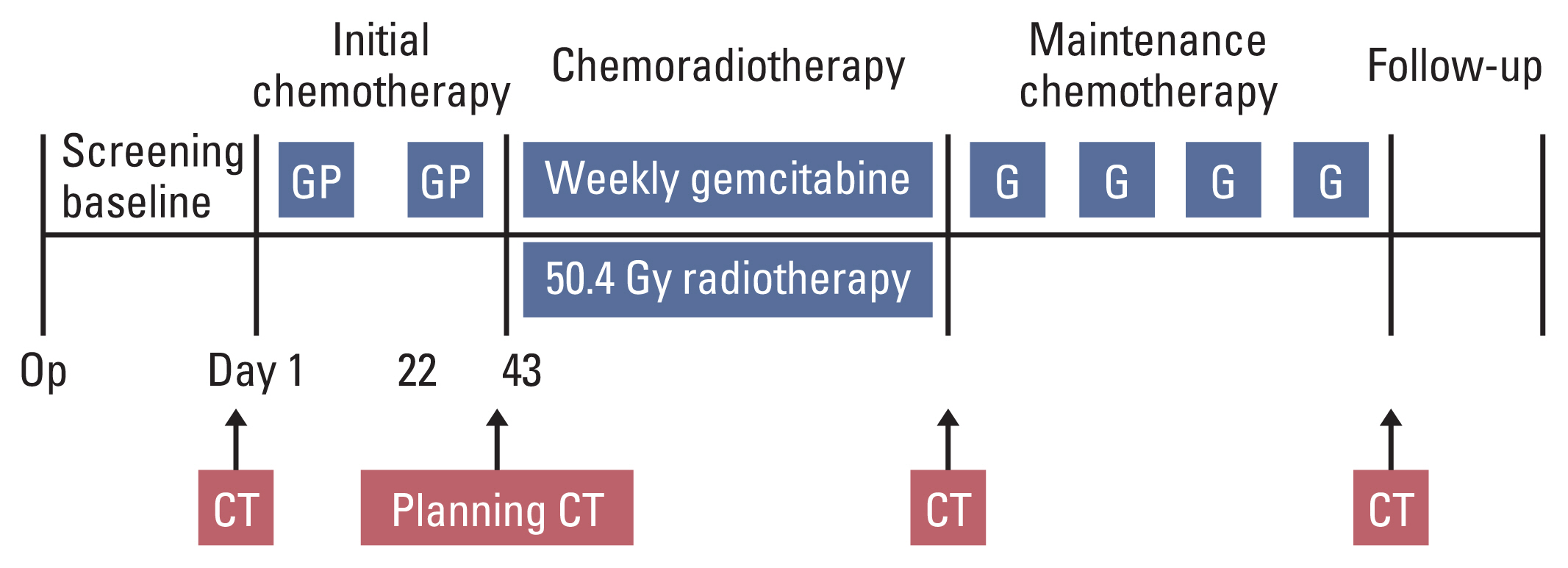
Patients with curatively resected stage IB-IIB pancreatic adenocarcinoma were eligible. Treatment consisted of chemotherapy with gemcitabine 1,000 mg/m2 on days 1 and 8 and cisplatin 60 mg/m2 on day 1 every 3 weeks for two cycles, followed by chemoradiotherapy (50.4 Gy/28 fx) with weekly gemcitabine (300 mg/m2/wk), and then gemcitabine 1,000 mg/m2 on days 1 and 8 every 3 weeks for four cycles. The primary endpoint was 1-year disease-free survival rate. The secondary endpoints were disease-free survival, overall survival, and safety.
Results
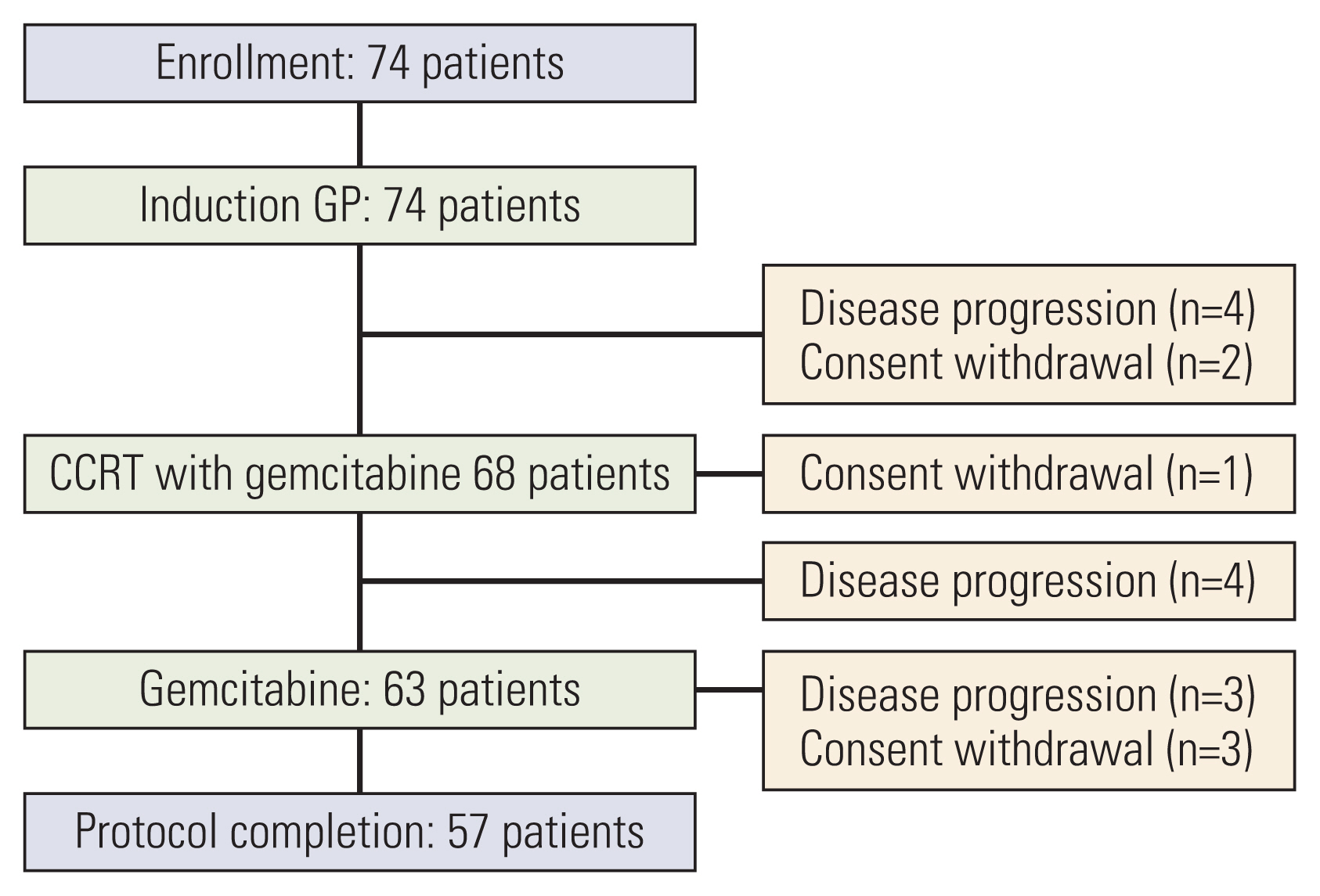
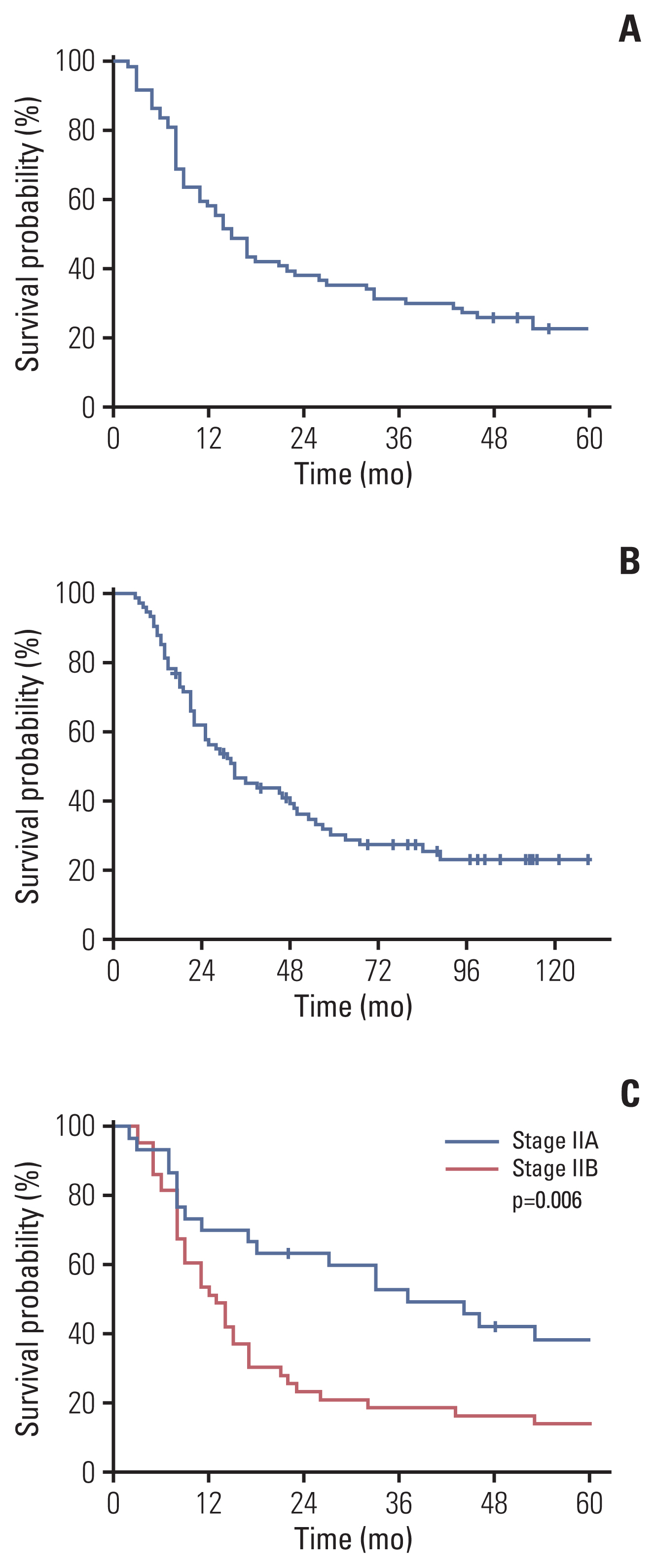
Seventy-four patients were enrolled. One-year disease-free survival rate was 57.9%. Median disease-free and overall survival were 15.0 months (95% confidence interval [CI], 11.6 to 18.4) and 33.0 months (95% CI, 21.8 to 44.2), respectively. At the median follow-up of 32 months, 57 patients (77.0%) had recurrence including 11 patients whose recurrence was during the adjuvant treatment. Most of the recurrences were systemic (52 patients). Stage at the time of diagnosis (70.0% in IIA, 51.2% in IIB, p=0.006) were significantly related with 1-year disease-free survival rate. Toxicities were generally tolerable, with 53 events of grade 3 or 4 hematologic toxicity and four patients with febrile neutropenia.
Conclusion
Adjuvant gemcitabine and cisplatin chemotherapy followed by chemoradiotherapy with gemcitabine and maintenance gemcitabine showed efficacy and good tolerability in curatively resected pancreatic cancer.
Figure
Reference
-
References
1. Saif MW. Controversies in the adjuvant treatment of pancreatic adenocarcinoma. JOP. 2007; 8:545–52.2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA Cancer J Clin. 2020; 70:7–30.
Article3. Kalser MH, Ellenberg SS. Pancreatic cancer: adjuvant combined radiation and chemotherapy following curative resection. Arch Surg. 1985; 120:899–903.4. Regine WF, Winter KA, Abrams RA, Safran H, Hoffman JP, Konski A, et al. Fluorouracil vs gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA. 2008; 299:1019–26.5. Neoptolemos JP, Stocken DD, Friess H, Bassi C, Dunn JA, Hickey H, et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N Engl J Med. 2004; 350:1200–10.
Article6. Katz MH, Fleming JB, Lee JE, Pisters PW. Current status of adjuvant therapy for pancreatic cancer. Oncologist. 2010; 15:1205–13.
Article7. Neoptolemos JP, Palmer DH, Ghaneh P, Psarelli EE, Valle JW, Halloran CM, et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet. 2017; 389:1011–24.
Article8. Uesaka K, Boku N, Fukutomi A, Okamura Y, Konishi M, Matsumoto I, et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet. 2016; 388:248–57.
Article9. Klinkenbijl JH, Jeekel J, Sahmoud T, van Pel R, Couvreur ML, Veenhof CH, et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann Surg. 1999; 230:776–82.10. Oettle H, Post S, Neuhaus P, Gellert K, Langrehr J, Ridwelski K, et al. Adjuvant chemotherapy with gemcitabine vs observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA. 2007; 297:267–77.11. Corsini MM, Miller RC, Haddock MG, Donohue JH, Farnell MB, Nagorney DM, et al. Adjuvant radiotherapy and chemotherapy for pancreatic carcinoma: the Mayo Clinic experience (1975–2005). J Clin Oncol. 2008; 26:3511–6.
Article12. Herman JM, Swartz MJ, Hsu CC, Winter J, Pawlik TM, Sugar E, et al. Analysis of fluorouracil-based adjuvant chemotherapy and radiation after pancreaticoduodenectomy for ductal adenocarcinoma of the pancreas: results of a large, prospectively collected database at the Johns Hopkins Hospital. J Clin Oncol. 2008; 26:3503–10.
Article13. Conroy T, Hammel P, Hebbar M, Ben Abdelghani M, Wei AC, Raoul JL, et al. FOLFIRINOX or gemcitabine as adjuvant therapy for pancreatic cancer. N Engl J Med. 2018; 379:2395–406.
Article14. Tempero MA, Reni M, Riess H, Pelzer U, O’Reilly EM, Winter JM, et al. APACT: phase III, multicenter, international, open-label, randomized trial of adjuvant nab-paclitaxel plus gemcitabine (nab-P/G) vs gemcitabine (G) for surgically resected pancreatic adenocarcinoma. J Clin Oncol. 2019; 37(15 Suppl):4000.
Article15. Liao WC, Chien KL, Lin YL, Wu MS, Lin JT, Wang HP, et al. Adjuvant treatments for resected pancreatic adenocarcinoma: a systematic review and network meta-analysis. Lancet Oncol. 2013; 14:1095–103.
Article16. National Comprehensive Cancer Network. Pancreatic adenocarcinoma, version 1. 2020 [Internet]. Plymouth Meeting, PA: National Comprehensive Cancer Network;2020. [cited 2020 Feb 20]. Available from: https://www2.tri-kobe.org/nccn/guideline/pancreas/english/pancreatic.pdf .17. Khorana AA, Mangu PB, Berlin J, Engebretson A, Hong TS, Maitra A, et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2016; 34:2541–56.
Article18. Peters GJ, Ruiz van Haperen VW, Bergman AM, Veerman G, Smitskamp-Wilms E, van Moorsel CJ, et al. Preclinical combination therapy with gemcitabine and mechanisms of resistance. Semin Oncol. 1996; 23(5 Suppl 10):16–24.19. Philip PA. Gemcitabine and platinum combinations in pancreatic cancer. Cancer. 2002; 95(4 Suppl):908–11.
Article20. Blackstock AW, Bernard SA, Richards F, Eagle KS, Case LD, Poole ME, et al. Phase I trial of twice-weekly gemcitabine and concurrent radiation in patients with advanced pancreatic cancer. J Clin Oncol. 1999; 17:2208–12.
Article21. Van Laethem JL, Demols A, Gay F, Closon MT, Collette M, Polus M, et al. Postoperative adjuvant gemcitabine and concurrent radiation after curative resection of pancreatic head carcinoma: a phase II study. Int J Radiat Oncol Biol Phys. 2003; 56:974–80.
Article22. Crane CH, Wolff RA, Abbruzzese JL, Evans DB, Milas L, Mason K, et al. Combining gemcitabine with radiation in pancreatic cancer: understanding important variables influencing the therapeutic index. Semin Oncol. 2001; 28(3 Suppl 10):25–33.
Article23. McGinn CJ, Lawrence TS, Zalupski MM. On the development of gemcitabine-based chemoradiotherapy regimens in pancreatic cancer. Cancer. 2002; 95(4 Suppl):933–40.
Article24. Colucci G, Giuliani F, Gebbia V, Biglietto M, Rabitti P, Uomo G, et al. Gemcitabine alone or with cisplatin for the treatment of patients with locally advanced and/or metastatic pancreatic carcinoma: a prospective, randomized phase III study of the Gruppo Oncologia dell’Italia Meridionale. Cancer. 2002; 94:902–10.25. Heinemann V, Quietzsch D, Gieseler F, Gonnermann M, Schonekas H, Rost A, et al. Randomized phase III trial of gemcitabine plus cisplatin compared with gemcitabine alone in advanced pancreatic cancer. J Clin Oncol. 2006; 24:3946–52.
Article26. Ducreux M, Rougier P, Pignon JP, Douillard JY, Seitz JF, Bugat R, et al. A randomised trial comparing 5-FU with 5-FU plus cisplatin in advanced pancreatic carcinoma. Ann Oncol. 2002; 13:1185–91.
Article27. Further evidence of effective adjuvant combined radiation and chemotherapy following curative resection of pancreatic cancer. Gastrointestinal Tumor Study Group. Cancer. 1987; 59:2006–10.28. Neoptolemos JP, Stocken DD, Bassi C, Ghaneh P, Cunningham D, Goldstein D, et al. Adjuvant chemotherapy with fluorouracil plus folinic acid vs gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA. 2010; 304:1073–81.29. Nie RC, Zou XB, Yuan SQ, Chen YB, Chen S, Chen YM, et al. Disease-free survival as a surrogate endpoint for overall survival in adjuvant trials of pancreatic cancer: a meta-analysis of 20 randomized controlled trials. BMC Cancer. 2020; 20:421.
Article30. Bilimoria KY, Bentrem DJ, Ko CY, Ritchey J, Stewart AK, Winchester DP, et al. Validation of the 6th edition AJCC Pancreatic Cancer Staging System: report from the National Cancer Database. Cancer. 2007; 110:738–44.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A Phase II Study of Combination Chemotherapy with Gemcitabine, 5-fluorouracil, and Cisplatin for Advanced Pancreatic Cancer
- Chemotherapy for Pancreatic Cancer
- Novel Palliative Chemotherapy for Cholangiocarcinoma
- Long Term Complete Response of Unresectable Locally Advanced Pancreatic Cancer after CCRT and Gemcitabine Chemotherapy
- Gemcitabine-based Chemotherapy for Gallbladder Cancer