Cancer Res Treat.
2021 Oct;53(4):1084-1095. 10.4143/crt.2020.1381.
Talazoparib Versus Chemotherapy in Patients with HER2-negative Advanced Breast Cancer and a Germline BRCA1/2 Mutation Enrolled in Asian Countries: Exploratory Subgroup Analysis of the Phase III EMBRACA Trial
- Affiliations
-
- 1Seoul National University Hospital, Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 2Yonsei Cancer Center, Yonsei University College of Medicine, Seoul, Korea
- 3Concord Repatriation General Hospital, Concord, NSW, Australia
- 4Pfizer, Milan, Italy
- 5Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2521583
- DOI: http://doi.org/10.4143/crt.2020.1381
Abstract
- Purpose
We evaluated study outcomes in patients enrolled in Asian regions in the phase III EMBRACA trial of talazoparib vs. chemotherapy.
Materials and Methods
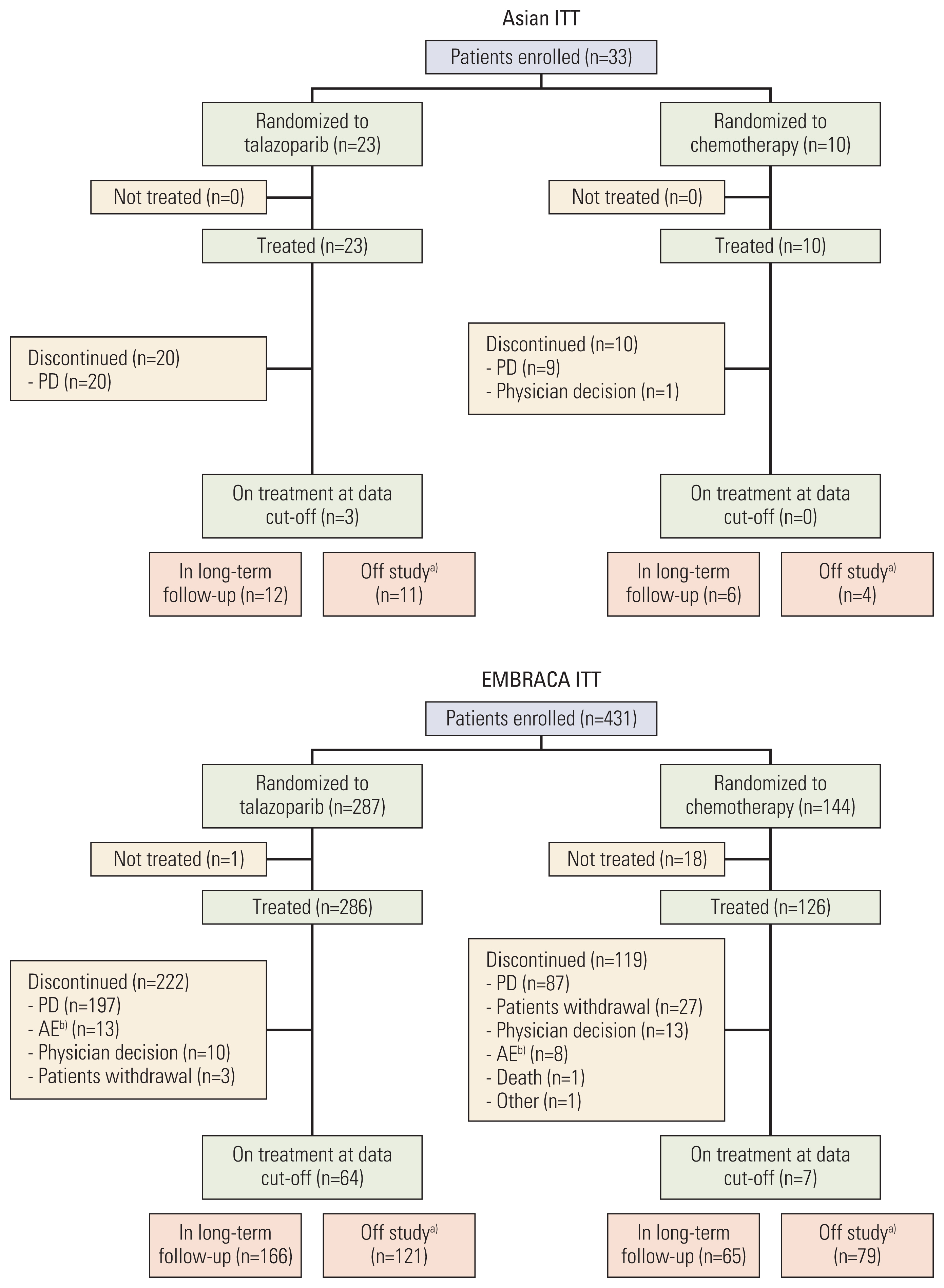
Patients with human epidermal growth factor receptor 2–negative germline BRCA1/2-mutated advanced breast cancer who received prior chemotherapy were randomized 2:1 to talazoparib 1 mg/day or chemotherapy (physician’s choice). Primary endpoint was progression-free survival (PFS) per independent central review in the intent-to-treat (ITT) population. This post-hoc analysis evaluated efficacy/safety endpoints in the ITT population of patients enrolled in Asian regions.
Results
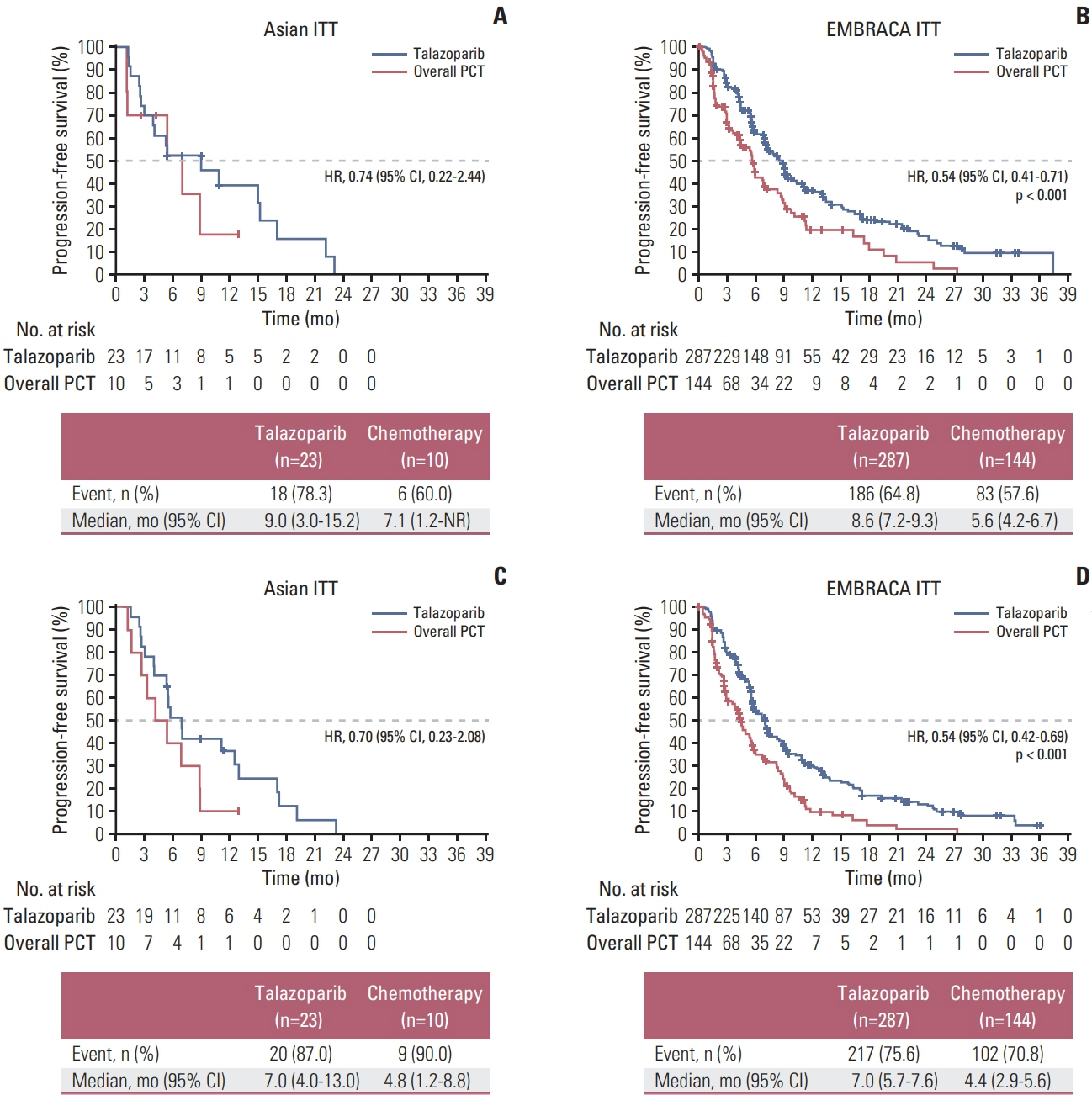
Thirty-three patients were enrolled at Asian sites (talazoparib, n=23; chemotherapy, n=10). Baseline characteristics were generally comparable with the overall EMBRACA population. In Asian patients, median PFS was 9.0 months (95% confidence interval [CI] 3.0, 15.2) for talazoparib and 7.1 months (95% CI, 1.2, not reached) for chemotherapy (hazard ratio [HR] 0.74 [95% CI, 0.22, 2.44]). Objective response rate was numerically higher for talazoparib vs. chemotherapy (62.5% [95% CI, 35.4, 84.8] vs. 25.0% [95% CI, 3.2, 65.1]). Median overall survival was 20.7 months (95% CI, 9.4, 40.1) versus 21.2 months (95% CI, 2.7, 35.0) months (HR, 1.41 [95% CI, 0.49, 4.05]). In Asian patients, fewer grade 3/4 adverse events (AEs), serious AEs (SAEs), grade 3/4 SAEs, and AEs resulting in dose reduction/discontinuation occurred with talazoparib than chemotherapy; for talazoparib, the frequency of these events was lower in Asian patients versus overall EMBRACA population.
Conclusion
In this subgroup analysis, talazoparib numerically improved efficacy versus chemotherapy and was generally well tolerated in Asian patients, with fewer grade 3/4 TEAEs, SAEs, and TEAEs leading to dose modification vs. the overall EMBRACA population.
Keyword
Figure
Reference
-
References
1. Javle M, Curtin NJ. The potential for poly (ADP-ribose) polymerase inhibitors in cancer therapy. Ther Adv Med Oncol. 2011; 3:257–67.
Article2. Ashworth A. A synthetic lethal therapeutic approach: poly (ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008; 26:3785–90.3. Helleday T. The underlying mechanism for the PARP and BRCA synthetic lethality: clearing up the misunderstandings. Mol Oncol. 2011; 5:387–93.
Article4. Lord CJ, Ashworth A. PARP inhibitors: Synthetic lethality in the clinic. Science. 2017; 355:1152–8.
Article5. Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012; 72:5588–99.
Article6. Murai J, Huang SY, Renaud A, Zhang Y, Ji J, Takeda S, et al. Stereospecific PARP trapping by BMN 673 and comparison with olaparib and rucaparib. Mol Cancer Ther. 2014; 13:433–43.
Article7. de Bono J, Ramanathan RK, Mina L, Chugh R, Glaspy J, Rafii S, et al. Phase I, dose-escalation, two-part trial of the PARP inhibitor talazoparib in patients with advanced germline BRCA1/2 mutations and selected sporadic cancers. Cancer Discov. 2017; 7:620–9.
Article8. Litton JK, Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018; 379:753–63.
Article9. U.S. Food and Drug Administration. TALZENNA (talazoparib) prescribing information [Internet]. New York: Pfizer;c2020. [cited 2020 Nov 19]. Available from: http://labeling.pfizer.com/ShowLabeling.aspx?id=11046 .10. European Medicines Agency. TALZENNA (talazoparib) Summary of product characteristics [Internet]. New York: Pfizer;c2019. [cited 2020 Nov 19]. Available from: https://www.ema.europa.eu/en/documents/product-information/talzenna-epar-product-information_en.pdf .11. Li G, Guo X, Tang L, Chen M, Luo X, Peng L, et al. Analysis of BRCA1/2 mutation spectrum and prevalence in unselected Chinese breast cancer patients by next-generation sequencing. J Cancer Res Clin Oncol. 2017; 143:2011–24.
Article12. Lang GT, Shi JX, Hu X, Zhang CH, Shan L, Song CG, et al. The spectrum of BRCA mutations and characteristics of BRCA-associated breast cancers in China: screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer. 2017; 141:129–42.
Article13. Kim H, Choi DH. Distribution of BRCA1 and BRCA2 mutations in Asian patients with breast cancer. J Breast Cancer. 2013; 16:357–65.14. Wen WX, Allen J, Lai KN, Mariapun S, Hasan SN, Ng PS, et al. Inherited mutations in BRCA1 and BRCA2 in an unselected multiethnic cohort of Asian patients with breast cancer and healthy controls from Malaysia. J Med Genet. 2018; 55:97–103.
Article15. Yap YS, Lu YS, Tamura K, Lee JE, Ko EY, Park YH, et al. Insights Into breast cancer in the East vs the West: a review. JAMA Oncol. 2019; 5:1489–96.16. Bhaskaran SP, Huang T, Rajendran BK, Guo M, Luo J, Qin Z, et al. Ethnic-specific BRCA1/2 variation within Asia population: evidence from over 78 000 cancer and 40,000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J Med Genet. 2020. Sep. 22. [Epub]. https://doi.org/10.1136/jmedgenet-2020-107299 .
Article17. Rugo HS, Ettl J, Hurvitz SA, Goncalves A, Lee KH, Fehrenbacher L, et al. Outcomes in clinically relevant patient subgroups from the EMBRACA study: talazoparib vs physician’s choice standard-of-care chemotherapy. JNCI Cancer Spectr. 2020; 4:pkz085.
Article18. Im SA, Xu B, Li W, Robson M, Ouyang Q, Yeh DC, et al. Olaparib monotherapy for Asian patients with a germline BRCA mutation and HER2-negative metastatic breast cancer: OlympiAD randomized trial subgroup analysis. Sci Rep. 2020; 10:8753.
Article19. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017; 377:523–33.
Article20. Lin CH, Yap YS, Lee KH, Im SA, Naito Y, Yeo W, et al. Contrasting epidemiology and clinicopathology of female breast cancer in Asians vs the US population. J Natl Cancer Inst. 2019; 111:1298–306.
Article21. Bhoo-Pathy N, Yip CH, Hartman M, Uiterwaal CS, Devi BC, Peeters PH, et al. Breast cancer research in Asia: adopt or adapt Western knowledge? Eur J Cancer. 2013; 49:703–9.
Article22. Hurvitz SA, Goncalves A, Rugo HS, Lee KH, Fehrenbacher L, Mina LA, et al. Talazoparib in patients with a germline BRCA-mutated advanced breast cancer: detailed safety analyses from the phase III EMBRACA trial. Oncologist. 2020; 25:e439–50.
Article23. Kan Z, Ding Y, Kim J, Jung HH, Chung W, Lal S, et al. Multi-omics profiling of younger Asian breast cancers reveals distinctive molecular signatures. Nat Commun. 2018; 9:1725.
Article24. Chen H, Wu J, Zhang Z, Tang Y, Li X, Liu S, et al. Association between BRCA status and triple-negative breast cancer: a meta-analysis. Front Pharmacol. 2018; 9:909.
Article25. Shi J, Liu F, Song Y. Progress: targeted therapy, immunotherapy, and new chemotherapy strategies in advanced triple-negative breast cancer. Cancer Manag Res. 2020; 12:9375–87.26. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Goncalves A, et al. Talazoparib versus chemotherapy in patients with germline BRCA1/2-mutated HER2-negative advanced breast cancer: final overall survival results from the EMBRACA trial. Ann Oncol. 2020; 31:1526–35.
Article27. Yu Y, Durairaj C, Shi H, Wang DD. Population pharmacokinetics of talazoparib in patients with advanced cancer. J Clin Pharmacol. 2020; 60:218–28.
Article28. Murthy P, Muggia F. PARP inhibitors: clinical development, emerging differences, and the current therapeutic issues. Cancer Drug Resist. 2019; 2:665–79.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Frequency of BRCA1 and BRCA2 Germline Mutations Detected by Protein Truncation Test and Cumulative Risks of Breast and Ovarian Cancer among Mutation Carriers in Japanese Breast Cancer Families
- A Study for Germline Mutation of BRCA1 in Early Onset Breast Cancer Patients
- A Study on BRCA1/2 Mutations, Hormone Status and HER-2 Status in Korean Women with Early-onset Breast Cancer
- Prevalence of BRCA1 and BRCA2 Germline Mutations in Breast Cancer Women of Multiple Ethnic Region in Northwest China
- Distribution of BRCA1 and BRCA2 Mutations in Asian Patients with Breast Cancer