J Korean Med Sci.
2021 Oct;36(39):e242. 10.3346/jkms.2021.36.e242.
Survival, Prognosis, and Clinical Feature of Refractory Myasthenia Gravis: a 15-year Nationwide Cohort Study
- Affiliations
-
- 1Department of Pharmacy, School of Pharmacy, Sungkyunkwan University, Suwon, Korea
- 2Marcus Institute for Aging Research, Hebrew SeniorLife and Harvard Medical School, Boston, MA, USA
- 3Department of Neurology, Neuroscience Research Institute, Seoul National University Medical Research Council, Seoul Metropolitan Boramae Medical Center, Seoul National University College of Medicine, Seoul, Korea
- 4Department of Clinical Research Design & Evaluation, SAIHST, Sungkyunkwan University, Seoul, Korea
- KMID: 2521181
- DOI: http://doi.org/10.3346/jkms.2021.36.e242
Abstract
- Background
Myasthenia gravis (MG) is a rare classic autoimmune disease where immunosuppressant therapies have been successful to reduce MG attributable mortality fairly well. However, patients with refractory MG (rMG) among the actively treated MG (aMG) are nonresponsive to conventional therapy and display high disease severity, which calls for further research. We aimed to determine survival, prognosis, and clinical feature of patients with rMG compared to non-rMG.
Methods
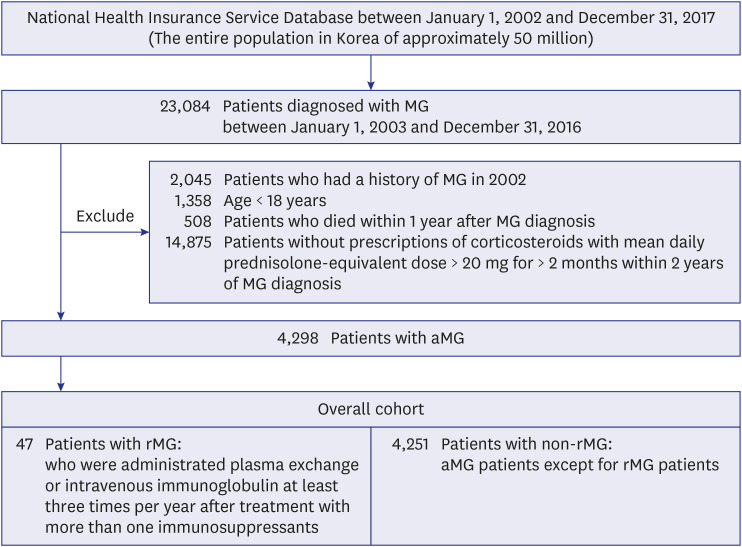
Retrospective nationwide cohort study using Korea's healthcare database between 2002 and 2017 was conducted. Patients with rMG (n = 47) and non-rMG (n = 4,251) who were aged > 18 years, followed-up for ≥ 1 year, and prescribed immunosuppressants within 2 years after incident MG diagnosis were included. Patients with rMG were defined as administered plasma exchange or intravenous immunoglobulin at least 3 times per year after receiving ≥ 2 immunosuppressants. All-cause mortality, myasthenic crisis, hospitalization, pneumonia/ sepsis, and emergency department (ED) visits were measured using Cox proportional hazard models and pharmacotherapy patterns for rMG were assessed.
Results
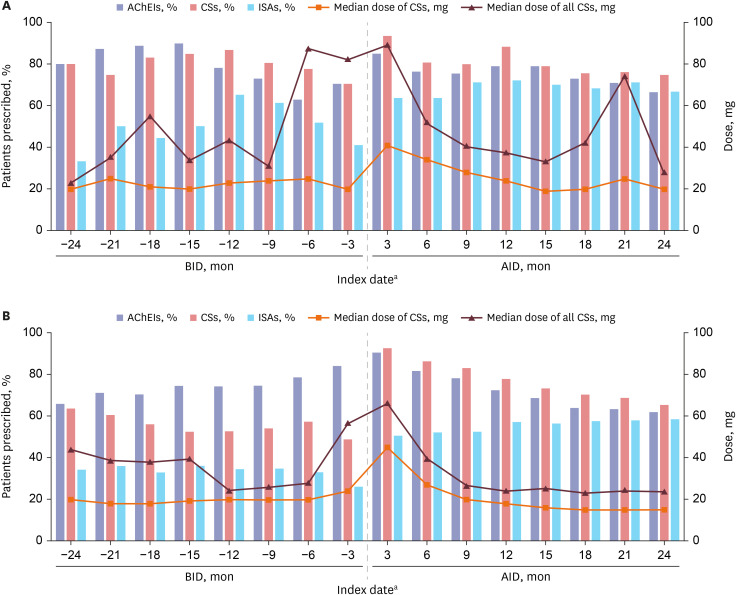
The rMG cohort included a preponderance of younger patients and women. The adjusted hazard ratio was 2.49 (95% confidence interval, 1.26–4.94) for mortality, 3.14 (2.25–4.38) for myasthenic crisis, 1.54 (1.15–2.06) for hospitalization, 2.69 (1.74–4.15) for pneumonia/sepsis, and 1.81 (1.28–2.56) for ED visits for rMG versus non-rMG. The immunosuppressant prescriptions were more prevalent in patients with rMG, while the difference was more remarkable before rMG onset rather than after rMG onset.
Conclusion
Despite the severe prognosis of rMG, the strategies for pharmacotherapeutic regimens were similar in those two groups, suggesting that intensive monitoring and introduction of timely treatment options in the early phase of MG are required.
Keyword
Figure
Reference
-
1. Dalakas MC. Novel future therapeutic options in myasthenia gravis. Autoimmun Rev. 2013; 12(9):936–941. PMID: 23537505.
Article2. National Institute on Neurological Disorders and Stroke. Myasthenia gravis fact sheet. Updated 2020. Accessed December 20, 2020. https://www.ninds.nih.gov/disorders/patient-caregiver-education/fact-sheets/Myasthenia-gravis-fact-sheet.3. Deenen JC, Horlings CG, Verschuuren JJ, Verbeek AL, van Engelen BG. The epidemiology of neuromuscular disorders: a comprehensive overview of the literature. J Neuromuscul Dis. 2015; 2(1):73–85. PMID: 28198707.
Article4. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016; 87(4):419–425. PMID: 27358333.5. Grob D, Brunner N, Namba T, Pagala M. Lifetime course of myasthenia gravis. Muscle Nerve. 2008; 37(2):141–149. PMID: 18059039.
Article6. Hansen JS, Danielsen DH, Somnier FE, Frøslev T, Jakobsen J, Johnsen SP, et al. Mortality in myasthenia gravis: a nationwide population-based follow-up study in Denmark. Muscle Nerve. 2016; 53(1):73–77. PMID: 25914186.
Article7. Owe JF, Daltveit AK, Gilhus NE. Causes of death among patients with myasthenia gravis in Norway between 1951 and 2001. J Neurol Neurosurg Psychiatry. 2006; 77(2):203–207. PMID: 16421123.
Article8. Sanders DB, Wolfe GI, Benatar M, Evoli A, Gilhus NE, Illa I, et al. International consensus guidance for management of myasthenia gravis: executive summary. Neurology. 2016; 87(4):419–425. PMID: 27358333.9. Cutter G, Xin H, Aban I, Burns TM, Allman PH, Farzaneh-Far R, et al. Cross-sectional analysis of the myasthenia gravis patient registry: disability and treatment. Muscle Nerve. 2019; 60(6):707–715. PMID: 31487038.
Article10. Hansen JS, Danielsen DH, Somnier FE, Frøslev T, Jakobsen J, Johnsen SP, et al. Mortality in myasthenia gravis: a nationwide population-based follow-up study in Denmark. Muscle Nerve. 2016; 53(1):73–77. PMID: 25914186.
Article11. Suh J, Goldstein JM, Nowak RJ. Clinical characteristics of refractory myasthenia gravis patients. Yale J Biol Med. 2013; 86(2):255–260. PMID: 23766745.12. Buzzard KA, Meyer NJ, Hardy TA, Riminton DS, Reddel SW. Induction intravenous cyclophosphamide followed by maintenance oral immunosuppression in refractory myasthenia gravis. Muscle Nerve. 2015; 52(2):204–210. PMID: 25487528.
Article13. Drachman DB, Adams RN, Hu R, Jones RJ, Brodsky RA. Rebooting the immune system with high-dose cyclophosphamide for treatment of refractory myasthenia gravis. Ann N Y Acad Sci. 2008; 1132(1):305–314. PMID: 18567882.
Article14. Lebrun C, Bourg V, Tieulie N, Thomas P. Successful treatment of refractory generalized myasthenia gravis with rituximab. Eur J Neurol. 2009; 16(2):246–250. PMID: 19146644.
Article15. Prakash KM, Ratnagopal P, Puvanendran K, Lo YL. Mycophenolate mofetil - as an adjunctive immunosuppressive therapy in refractory myasthenia gravis: the Singapore experience. J Clin Neurosci. 2007; 14(3):278–281. PMID: 16597503.
Article16. Gladstone DE, Brannagan TH 3rd, Schwartzman RJ, Prestrud AA, Brodsky I. High dose cyclophosphamide for severe refractory myasthenia gravis. J Neurol Neurosurg Psychiatry. 2004; 75(5):789–791.
Article17. Lee J, Lee JS, Park SH, Shin SA, Kim K. Cohort profile: The National Health Insurance Service-National Sample Cohort (NHIS-NSC), South Korea. Int J Epidemiol. 2017; 46(2):e15. PMID: 26822938.
Article18. Kinnett K, Noritz G. The PJ Nicholoff steroid protocol for Duchenne and Becker muscular dystrophy and adrenal suppression. PLoS Curr. 2017; 9:9.19. Quan H, Li B, Couris CM, Fushimi K, Graham P, Hider P, et al. Updating and validating the Charlson comorbidity index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011; 173(6):676–682. PMID: 21330339.
Article20. Gilhus NE, Nacu A, Andersen JB, Owe JF. Myasthenia gravis and risks for comorbidity. Eur J Neurol. 2015; 22(1):17–23. PMID: 25354676.
Article21. Lai CH, Tseng HF. Nationwide population-based epidemiological study of myasthenia gravis in Taiwan. Neuroepidemiology. 2010; 35(1):66–71. PMID: 20523074.
Article22. Baggi F, Andreetta F, Maggi L, Confalonieri P, Morandi L, Salerno F, et al. Complete stable remission and autoantibody specificity in myasthenia gravis. Neurology. 2013; 80(2):188–195. PMID: 23255823.
Article23. Somnier FE, Keiding N, Paulson OB. Epidemiology of myasthenia gravis in Denmark. A longitudinal and comprehensive population survey. Arch Neurol. 1991; 48(7):733–739. PMID: 1859301.
Article24. Wolfe GI, Kaminski HJ, Aban IB, Minisman G, Kuo HC, Marx A, et al. Randomized trial of thymectomy in myasthenia gravis. N Engl J Med. 2016; 375(6):511–522. PMID: 27509100.
Article25. Engel-Nitz NM, Boscoe A, Wolbeck R, Johnson J, Silvestri NJ. Burden of illness in patients with treatment refractory myasthenia gravis. Muscle Nerve. 2018; 58(11):99–105.
Article26. Liu C, Wang Q, Qiu Z, Lin J, Chen B, Li Y, et al. Analysis of mortality and related factors in 2195 adult myasthenia gravis patients in a 10-year follow-up study. Neurol India. 2017; 65(3):518–524. PMID: 28488612.
Article27. Murai H, Hasebe M, Murata T, Utsugisawa K. Clinical burden and healthcare resource utilization associated with myasthenia gravis: assessments from a Japanese claims database. Clin Exp Neuroimmunol. 2019; 10(1):61–68.
Article28. Mantegazza R, Antozzi C. When myasthenia gravis is deemed refractory: clinical signposts and treatment strategies. Ther Adv Neurol Disorder. 2018; 11:1756285617749134.
Article29. Silvestri NJ, Wolfe GI. Rituximab in treatment-refractory myasthenia gravis. JAMA Neurol. 2017; 74(1):21–23. PMID: 27892981.
Article30. Topakian R, Zimprich F, Iglseder S, Embacher N, Guger M, Stieglbauer K, et al. High efficacy of rituximab for myasthenia gravis: a comprehensive nationwide study in Austria. J Neurol. 2019; 266(3):699–706. PMID: 30649616.
Article31. Rollins SA, Sims PJ. The complement-inhibitory activity of CD59 resides in its capacity to block incorporation of C9 into membrane C5b-9. J Immunol. 1990; 144(9):3478–3483. PMID: 1691760.32. Rother RP, Rollins SA, Mojcik CF, Brodsky RA, Bell L. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007; 25(11):1256–1264. PMID: 17989688.
Article33. Brauner S, Eriksson-Dufva A, Hietala MA, Frisell T, Press R, Piehl F. Comparison between rituximab treatment for new-onset generalized myasthenia gravis and refractory generalized myasthenia gravis. JAMA Neurol. 2020; 77(8):974–981. PMID: 32364568.
Article34. Alexion Pharmaceuticals Inc. Soliris (eculizumab): US prescribing information. Updated 2015. Accessed November 13, 2017. https://alexion.com/Documents/Soliris_USPI.pdf.35. Alexion Europe SAS. Soliris (eculizumab): summary of product characteristics. Updated 2017. Accessed November 17, 2017. http://www.ema.europa.eu/docs/en_GB/ document_library/EPAR_-_Product_Information/human/000791/WC500054208.pdf.36. Japan Ministry of Health Labour and Welfare. Soliris (eculizumab): Japanese prescribing information. Updated 2017. Accessed January 10, 2018. https://www.mhlw.go.jp/shingi/2007/07/dl/s0730-17j.pdf.37. Howard JF Jr, Barohn RJ, Cutter GR, Freimer M, Juel VC, Mozaffar T, et al. A randomized, double-blind, placebo-controlled phase II study of eculizumab in patients with refractory generalized myasthenia gravis. Muscle Nerve. 2013; 48(1):76–84. PMID: 23512355.
Article38. Howard JF Jr, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Safety and efficacy of eculizumab in anti-acetylcholine receptor antibody-positive refractory generalised myasthenia gravis (REGAIN): a phase 3, randomised, double-blind, placebo-controlled, multicentre study. Lancet Neurol. 2017; 16(12):976–986. PMID: 29066163.39. Muppidi S, Utsugisawa K, Benatar M, Murai H, Barohn RJ, Illa I, et al. Long-term safety and efficacy of eculizumab in generalized myasthenia gravis. Muscle Nerve. 2019; 60(1):14–24. PMID: 30767274.40. Andersen H, Mantegazza R, Wang JJ, O'Brien F, Patra K, Howard JF Jr, et al. Eculizumab improves fatigue in refractory generalized myasthenia gravis. Qual Life Res. 2019; 28(8):2247–2254. PMID: 30905021.