J Liver Cancer.
2021 Sep;21(2):146-154. 10.17998/jlc.2021.05.20.
Transarterial chemoembolization using drug-eluting bead compared with radiofrequency ablation for treatment of single small hepatocellular carcinoma: a pilot non-randomized trial
- Affiliations
-
- 1Department of Internal Medicine, Cheju Halla General Hospital, Jeju, Korea
- 2Department of Internal Medicine, Yonsei University Medical College, Seoul, Korea
- 3Department of Radiology, Cheju Halla General Hospital, Jeju, Korea
- KMID: 2520901
- DOI: http://doi.org/10.17998/jlc.2021.05.20
Abstract
- Background/Aims
Surgical resection, transplantation, and radiofrequency ablation (RFA) are generally accepted as amenable treatments for small hepatocellular carcinoma (HCC). Recently drug-eluting beads (DEB) which had several treatment advantages were introduced for transarterial chemoembolization (TACE). The aim of this study was to evaluate feasibility and safety of DEB-TACE compared with RFA for the treatment of single small HCC.
Methods
In this pilot non-randomized trial, we assessed retrospective data of 40 patients who underwent DEB-TACE (n=21) or RFA (n=19) for single small (≤3 centimeter in greatest dimension) HCC. The primary outcomes were tumor response and time to recurrence. The secondary outcome was treatment-related complications.
Results
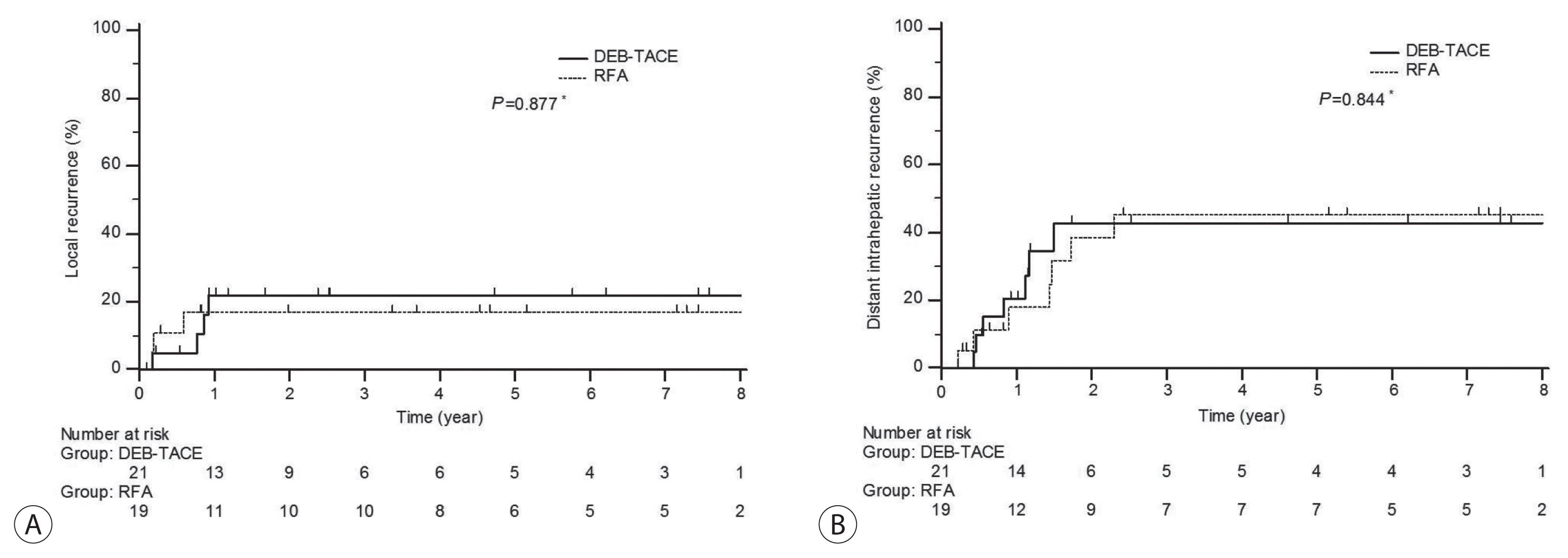
Complete response rate to DEB-TACE and RFA after first follow-up assessment was 90.5% and 94.7%, respectively (P=1.000). During mean follow-up of 87.6 months (95% confidence interval: 74.4-102), 7 patients experienced local recurrence. The 6- and 12-month cumulative local recurrence rate was 5.0% and 21.8% in DEB-TACE vs. 11.1% and 17.0% in RFA group (P=0.877). A total 14 distant intrahepatic recurrences were developed and 12- and 24-month cumulative distant intrahepatic recurrence rate was 20.6% and 42.7% in DEBTACE vs. 17.2% and 36.3% in RFA group (P=0.844). Two patients experienced gangrenous cholecystitis after DEB-TACE requiring cholecystectomy as treatment-related adverse event.
Conclusions
Tumor response and recurrence rate after single session of DEB-TACE or RFA were similar. DEB-TACE could be applied selectively in patients with a single small HCC if the other therapeutic modality is unfeasible.
Keyword
Figure
Reference
-
References
1. Perz JF, Armstrong GL, Farrington LA, Hutin YJ, Bell BP. The contributions of hepatitis B virus and hepatitis C virus infections to cirrhosis and primary liver cancer worldwide. J Hepatol. 2006; 45:529–538.2. Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. Management of hepatocellular carcinoma. Hepatology. 2005; 42:1208–1236.3. Llovet JM, Di Bisceglie AM, Bruix J, Kramer BS, Lencioni R, Zhu AX, et al. Design and endpoints of clinical trials in hepatocellular carcinoma. J Natl Cancer Inst. 2008; 100:698–711.4. Bruix J, Sala M, Llovet JM. Chemoembolization for hepatocellular carcinoma. Gastroenterology. 2004; 127(5 Suppl 1):S179–S188.5. Lencioni R. Loco-regional treatment of hepatocellular carcinoma. Hepatology. 2010; 52:762–773.6. Hong K, Khwaja A, Liapi E, Torbenson MS, Georgiades CS, Geschwind JF. New intra-arterial drug delivery system for the treatment of liver cancer: preclinical assessment in a rabbit model of liver cancer. Clin Cancer Res. 2006; 12:2563–2567.7. Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, McFadden ET, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982; 5:649–655.8. Heimbach JK, Kulik LM, Finn RS, Sirlin CB, Abecassis MM, Roberts LR, et al. AASLD guidelines for the treatment of hepatocellular carcinoma. Hepatology. 2018; 67:358–380.9. European Association for the Study of the Liver. EASL clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2018; 69:182–236.10. Ahn SS, Kim MJ, Lim JS, Hong HS, Chung YE, Choi JY. Added value of gadoxetic acid-enhanced hepatobiliary phase MR imaging in the diagnosis of hepatocellular carcinoma. Radiology. 2010; 255:459–466.11. Doyle A, Gorgen A, Muaddi H, Aravinthan AD, Issachar A, Mironov O, et al. Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol. 2019; 70:866–873.12. Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010; 30:52–60.13. National Cancer Institute. Common terminology criteria for adverse events (CTCAE) version 4.0. 2009. [Internet]. Bethesda (MD): National Cancer Institute;[cited 2020 Nov 14]. Available from: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03/CT-CAE_4.03_2010-06-14_QuickReference_5x7.pdf .14. Marrero JA, Kulik LM, Sirlin CB, Zhu AX, Finn RS, Abecassis MM, et al. Diagnosis, staging, and management of hepatocellular carcinoma: 2018 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2018; 68:723–750.15. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int. 2017; 11:317–370.16. Korean Liver Cancer Association; National Cancer Center. 2018 Korean Liver Cancer Association-National Cancer Center Korea practice guidelines for the management of hepatocellular carcinoma. Gut Liver. 2019; 13:227–299.17. Nault JC, Sutter O, Nahon P, Ganne-Carrié N, Séror O. Percutaneous treatment of hepatocellular carcinoma: state of the art and innovations. J Hepatol. 2018; 68:783–797.18. Brunello F, Cantamessa A, Gaia S, Carucci P, Rolle E, Castiglione A, et al. Radiofrequency ablation: technical and clinical long-term outcomes for single hepatocellular carcinoma up to 30 mm. Eur J Gastroenterol Hepatol. 2013; 25:842–849.19. Francica G, Saviano A, De Sio I, De Matthaeis N, Brunello F, Cantamessa A, et al. Long-term effectiveness of radiofrequency ablation for solitary small hepatocellular carcinoma: a retrospective analysis of 363 patients. Dig Liver Dis. 2013; 45:336–341.20. Varela M, Real MI, Burrel M, Forner A, Sala M, Brunet M, et al. Chemoembolization of hepatocellular carcinoma with drug eluting beads: efficacy and doxorubicin pharmacokinetics. J Hepatol. 2007; 46:474–481.21. Poon RT, Tso WK, Pang RW, Ng KK, Woo R, Tai KS, et al. A phase I/II trial of chemoembolization for hepatocellular carcinoma using a novel intra-arterial drug-eluting bead. Clin Gastroenterol Hepatol. 2007; 5:1100–1108.22. Lee KH, Joo SM, Yum TJ, Jung SH. Conventional versus drug-eluting beads trans-arterial chemoembolization for treatment of hepatocellular carcinoma at very early and early stages. J Liver Cancer. 2017; 17:144–152.23. Lammer J, Malagari K, Vogl T, Pilleul F, Denys A, Watkinson A, et al. Prospective randomized study of doxorubicin-eluting-bead embolization in the treatment of hepatocellular carcinoma: results of the PRECISION V study. Cardiovasc Intervent Radiol. 2010; 33:41–52.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Drug-Eluting Bead Transarterial Chemoembolization Versus Radiofrequency Ablation as an Initial Treatment of Single Small (≤ 3 cm) Hepatocellular Carcinoma
- The Role of Combination of Transarterial Chemoebolization and Radiofrequency Ablation for Hepatocellular Carcinoma Treatment
- Complications Related to Transarterial Treatment of Hepatocellular Carcinoma: A Comprehensive Review
- Transarterial chemoembolization using drug eluting beads for the treatment of hepatocellular carcinoma: Now and future
- Efficacy of Hepatic Arterial Infusion Chemotherapy and Radiofrequency Ablation against Hepatocellular Carcinoma Refractory to Transarterial Chemoembolization and Vascular Variation: A Case Study