Korean J Transplant.
2021 Sep;35(3):168-176. 10.4285/kjt.21.0012.
Efficacy and safety of a switch from twicedaily tacrolimus to once-daily generic tacrolimus in stable liver transplant patients
- Affiliations
-
- 1Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Surgery, Korea University College of Medicine, Seoul, Korea
- 3Department of Surgery, Korea University Guro Hospital, Seoul, Korea
- 4Department of Surgery, Ewha Woman's University School of Medicine, Seoul, Korea
- 5Department of Surgery, Yonsei University College of Medicine, Seoul, Korea
- 6Department of Surgery, Dong-A University Hospital, Dong-A University College of Medicine, Busan, Korea
- 7Department of Surgery, Pusan National University College of Medicine, Busan, Korea
- 8Department of Surgery, Kyungpook National University Hospital, School of Medicine, Kyungpook National University, Daegu, Korea
- 9Department of Surgery, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Korea
- 10Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 11Division of Hepatobiliary Surgery and Liver Transplantation, Department of Surgery, Ajou University School of Medicine, Suwon, Korea
- 12Center for Liver Cancer, National Cancer Center, Goyang, Korea
- 13Department of Surgery, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea
- 14Department of Surgery, College of Medicine, The Catholic University of Korea, Seoul, Korea
- 15Department of Surgery, Seoul National University College of Medicine, Seoul, Korea
- 16Department of Surgery, Ulsan University Hospital, University of Ulsan College of Medicine, Ulsan, Korea
- 17Department of Surgery, Keimyung University Dongsan Hospital, Keimyung University School of Medicine, Daegu, Korea
- KMID: 2520678
- DOI: http://doi.org/10.4285/kjt.21.0012
Abstract
- Background
Once-daily tacrolimus reduces non-compliance relative to twice-daily tacrolimus. However, little is known about the safety and efficacy of conversion from twice-daily tacrolimus to generic once-daily tacrolimus in liver transplantation (LT). Herein, we investigated the efficacy and safety of a switch from twice-daily tacrolimus to generic once-daily tacrolimus in patients with stable liver graft function.
Methods
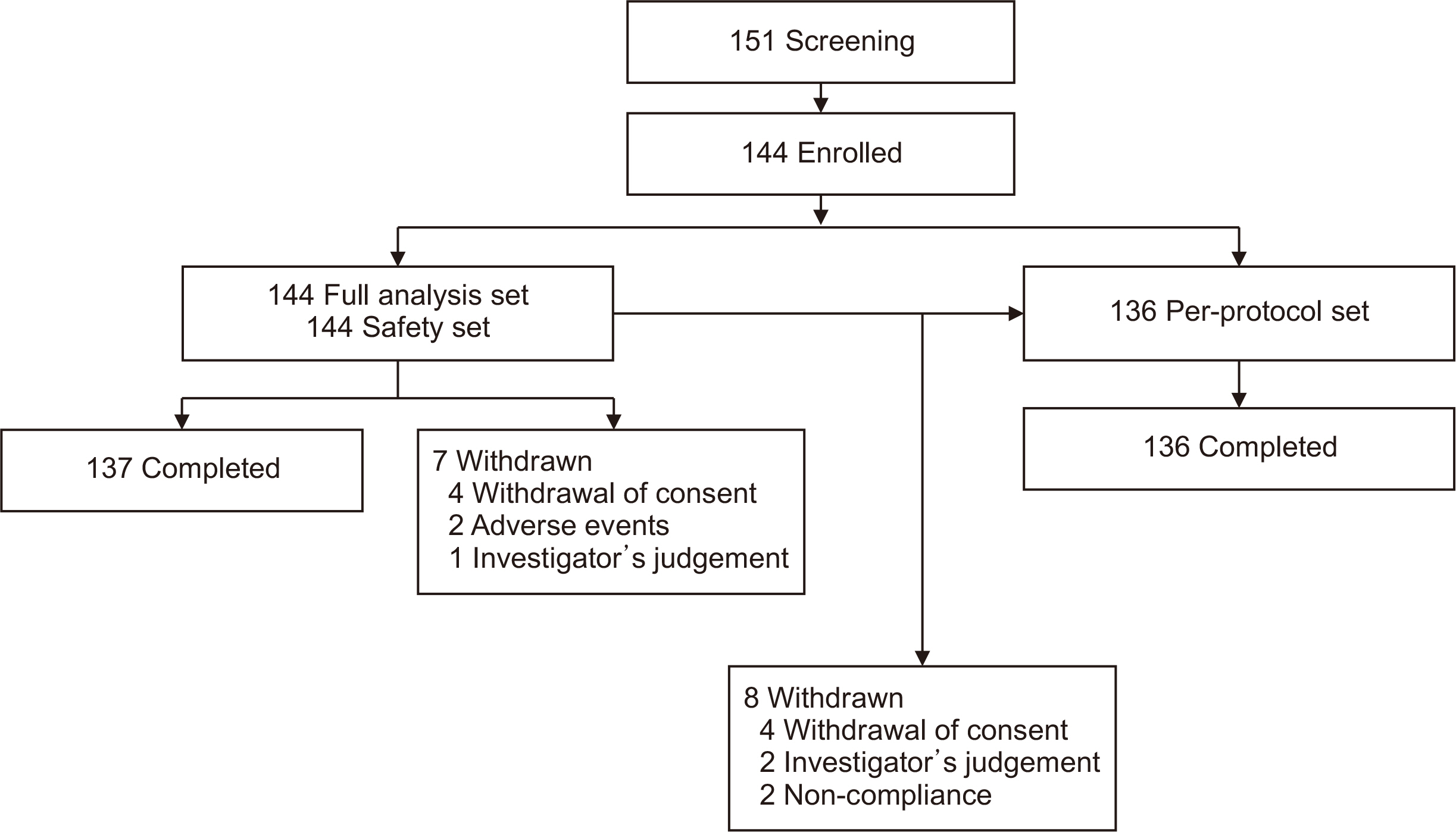
This prospective, multicenter, open-label, single-arm study was conducted in 17 medical centers for 1 year from July 2019 to July 2020 (NCT04069065). Primary endpoint was the incidence of biopsy-proven acute rejection (BPAR) for 24 weeks after conversion. Secondary endpoints were graft failure, patient death, and adverse events (AEs).
Results
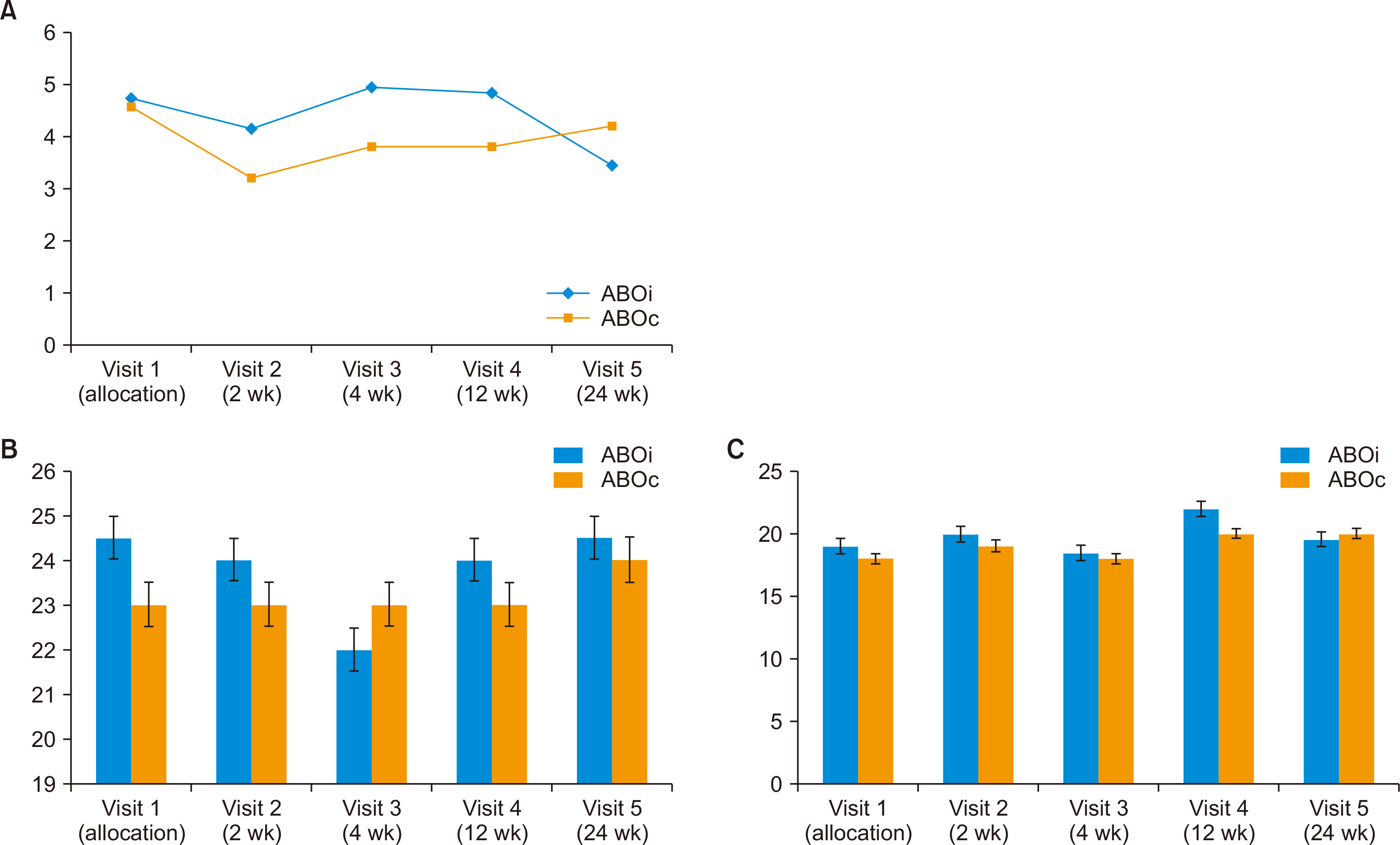
Of 151 screened LT patients, 144 patients were enrolled. BPAR, graft failure, and patient death did not occur in this patient population. There were no statistical differences in blood tests, liver function tests, or biochemical tests between visits in any of the patients. Median tacrolimus trough level decreased abruptly from 4.7 ng/mL to 3.2 ng/mL after generic once-daily tacrolimus conversion, but median tacrolimus dose increased due to low tacrolimus trough level. Ninety-two adverse events occurred in 54 patients. Liver enzyme levels increased in seven patients (4.9%) after the switch to generic once-daily tacrolimus, but the liver function tests of these patients normalized thereafter. There were three cases of severe AEs not related to investigational drug.
Conclusions
Present study suggests that conversion from twice-daily tacrolimus to generic once-daily tacrolimus is effective and safe in stable LT patients.
Figure
Reference
-
1. U.S. Multicenter FK506 Liver Study Group. 1994; A comparison of tacrolimus (FK 506) and cyclosporine for immunosuppression in liver transplantation. N Engl J Med. 331:1110–5. DOI: 10.1056/NEJM199410273311702. PMID: 7523946.2. Kuypers DRJ. 2020; Intrapatient variability of tacrolimus exposure in solid organ transplantation: a novel marker for clinical outcome. Clin Pharmacol Ther. 107:347–58. DOI: 10.1002/cpt.1618. PMID: 31449663.
Article3. Brunet M, van Gelder T, Åsberg A, Haufroid V, Hesselink DA, Langman L, et al. 2019; Therapeutic drug monitoring of tacrolimus-personalized therapy: second consensus report. Ther Drug Monit. 41:261–307. DOI: 10.1097/FTD.0000000000000640. PMID: 31045868.
Article4. Kuypers DR, Peeters PC, Sennesael JJ, Kianda MN, Vrijens B, Kristanto P, et al. 2013; Improved adherence to tacrolimus once-daily formulation in renal recipients: a randomized controlled trial using electronic monitoring. Transplantation. 95:333–40. DOI: 10.1097/TP.0b013e3182725532. PMID: 23263559.5. Fine RN, Becker Y, De Geest S, Eisen H, Ettenger R, Evans R, et al. 2009; Nonadherence consensus conference summary report. Am J Transplant. 9:35–41. DOI: 10.1111/j.1600-6143.2008.02495.x. PMID: 19133930.
Article6. Caillard S, Moulin B, Buron F, Mariat C, Audard V, Grimbert P, et al. 2016; Advagraf(®), a once-daily prolonged release tacrolimus formulation, in kidney transplantation: literature review and guidelines from a panel of experts. Transpl Int. 29:860–9. DOI: 10.1111/tri.12674. PMID: 26373896.
Article7. Iwasaki M, Yano I, Fukatsu S, Hashi S, Yamamoto Y, Sugimoto M, et al. 2018; Pharmacokinetics and pharmacodynamics of once-daily tacrolimus compared with twice-daily tacrolimus in the early stage after living donor liver transplantation. Ther Drug Monit. 40:675–81. DOI: 10.1097/FTD.0000000000000551. PMID: 29965882.
Article8. Kim JM, Ryu JH, Lee KW, Hong SK, Yang K, Choi GS, et al. 2020; Effect of CYP3A5 on the once-daily tacrolimus conversion in stable liver transplant patients. J Clin Med. 9:2897. DOI: 10.3390/jcm9092897. PMID: 32911703. PMCID: PMC7563461.9. Kim JM, Kwon CH, Joh JW, Sinn DH, Lee S, Choi GS, et al. 2016; Conversion of once-daily extended-release tacrolimus is safe in stable liver transplant recipients: a randomized prospective study. Liver Transpl. 22:209–16. DOI: 10.1002/lt.24336. PMID: 26360125.
Article10. Suh SW, Lee KW, Jeong J, Kim H, Yi NJ, Suh KS. 2016; Risk factors for the adverse events after conversion from twice-daily to once-daily tacrolimus in stable liver transplantation patients. J Korean Med Sci. 31:1711–6. DOI: 10.3346/jkms.2016.31.11.1711. PMID: 27709847. PMCID: PMC5056201.
Article11. Kim SH, Lee SD, Kim YK, Park SJ. 2015; Conversion of twice-daily to once-daily tacrolimus is safe in stable adult living donor liver transplant recipients. Hepatobiliary Pancreat Dis Int. 14:374–9. DOI: 10.1016/S1499-3872(15)60378-2.
Article12. Molnar AO, Fergusson D, Tsampalieros AK, Bennett A, Fergusson N, Ramsay T, et al. 2015; Generic immunosuppression in solid organ transplantation: systematic review and meta-analysis. BMJ. 350:h3163. DOI: 10.1136/bmj.h3163. PMID: 26101226. PMCID: PMC4476317.
Article13. Taube D, Jones G, O'Beirne J, Wennberg L, Connor A, Rasmussen A, et al. 2014; Generic tacrolimus in solid organ transplantation. Clin Transplant. 28:623–32. DOI: 10.1111/ctr.12336. PMID: 24750309.
Article14. Spence MM, Nguyen LM, Hui RL, Chan J. 2012; Evaluation of clinical and safety outcomes associated with conversion from brand-name to generic tacrolimus in transplant recipients enrolled in an integrated health care system. Pharmacotherapy. 32:981–7. DOI: 10.1002/phar.1130. PMID: 23074134.
Article15. Yu YD, Lee SG, Joh JW, Kwon CH, Kim DG, Suh KS, et al. 2012; Results of a phase 4 trial of Tacrobell® in liver transplantation patients: a multicenter study in South Korea. Hepatogastroenterology. 59:357–63. DOI: 10.5754/hge11472.
Article16. Son SY, Jang HR, Lee JE, Yoo H, Kim K, Park JB, et al. 2017; Comparison of the long-term efficacy and safety of generic Tacrobell with original tacrolimus (Prograf) in kidney transplant recipients. Drug Des Devel Ther. 11:203–10. DOI: 10.2147/DDDT.S118154. PMID: 28138224. PMCID: PMC5238812.
Article17. Park K, Kim YS, Kwon KI, Park MS, Lee YJ, Kim KH. 2007; A randomized, open-label, two-period, crossover bioavailability study of two oral formulations of tacrolimus in healthy Korean adults. Clin Ther. 29:154–62. DOI: 10.1016/j.clinthera.2007.01.016. PMID: 17379055.
Article18. Kim JM, Joh JW, Choi GS, Lee SK. 2019; Generic tacrolimus (Tacrobell ®) shows comparable outcomes to brand-name tacrolimus in the long-term period after adult deceased donor liver transplantation. Drug Des Devel Ther. 13:4431–8. DOI: 10.2147/DDDT.S229114. PMID: 32021085. PMCID: PMC6948198.19. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. 1999; A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med. 130:461–70. DOI: 10.7326/0003-4819-130-6-199903160-00002. PMID: 10075613.
Article20. TruneČka P, Klempnauer J, Bechstein WO, Pirenne J, Friman S, Zhao A, et al. 2015; Renal function in de novo liver transplant recipients receiving different prolonged-release tacrolimus regimens-the DIAMOND Study. Am J Transplant. 15:1843–54. DOI: 10.1111/ajt.13182. PMID: 25707487. PMCID: PMC5024030.21. Fischer L, Trunečka P, Gridelli B, Roy A, Vitale A, Valdivieso A, et al. 2011; Pharmacokinetics for once-daily versus twice-daily tacrolimus formulations in de novo liver transplantation: a randomized, open-label trial. Liver Transpl. 17:167–77. DOI: 10.1002/lt.22211. PMID: 21280190.
Article22. Beckebaum S, Iacob S, Sweid D, Sotiropoulos GC, Saner F, Kaiser G, et al. 2011; Efficacy, safety, and immunosuppressant adherence in stable liver transplant patients converted from a twice-daily tacrolimus-based regimen to once-daily tacrolimus extended-release formulation. Transpl Int. 24:666–75. DOI: 10.1111/j.1432-2277.2011.01254.x. PMID: 21466596.
Article23. Schutte-Nutgen K, Tholking G, Suwelack B, Reuter S. 2018; Tacrolimus: pharmacokinetic considerations for clinicians. Curr Drug Metab. 19:342–50. DOI: 10.2174/1389200219666180101104159. PMID: 29298646.24. Al Ameri MN, Whittaker C, Tucker A, Yaqoob M, Johnston A. 2011; A survey to determine the views of renal transplant patients on generic substitution in the UK. Transpl Int. 24:770–9. DOI: 10.1111/j.1432-2277.2011.01268.x. PMID: 21575080.
Article25. Alloway RR, Vinks AA, Fukuda T, Mizuno T, King EC, Zou Y, et al. 2017; Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: a randomized, crossover clinical trial. PLoS Med. 14:e1002428. DOI: 10.1371/journal.pmed.1002428. PMID: 29135993. PMCID: PMC5685573.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Modified Release Tacrolimus
- Risk of graft loss on once-daily versus twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- The comparison of coefficient of variation among once-daily and twice-daily tacrolimus in kidney transplant patients: a meta-analysis
- Single Center Experiences of Conversion from Twice-daily Tacrolimus (Prograf) to Once-daily Tacrolimus (Advagraf) in Stable Liver Transplant Recipients
- Evaluation of the efficacy and safety of conversion from the tacrolimus capsule to tablet in stable liver transplant recipients with maintenance therapy: a 24-week, open-label, single-center, phase IV exploratory clinical study