J Korean Med Sci.
2021 Sep;36(36):e222. 10.3346/jkms.2021.36.e222.
Waning Effectiveness of One-dose Universal Varicella Vaccination in Korea, 2011–2018: a Propensity Score Matched National Population Cohort
- Affiliations
-
- 1Department of Preventive Medicine, Korea University College of Medicine, Seoul, Korea
- 2Department of Pediatrics, Korea University Anam Hospital, Seoul, Korea
- 3Department of Pediatrics, Incheon Sejong Hospital, Incheon, Korea
- 4Department of Pediatrics, Chungbuk National University Hospital, Cheongju, Korea
- 5Department of Pediatrics, Seoul Metropolitan Government - Seoul National University Boramae Medical Center, Seoul, Korea
- 6Department of Pediatrics, Seoul National University Hospital, Seoul, Korea
- 7Department of Pediatrics, Seoul National University College of Medicine, Seoul, Korea
- KMID: 2520107
- DOI: http://doi.org/10.3346/jkms.2021.36.e222
Abstract
- Background
Despite high coverage (~98%) of universal varicella vaccination (UVV) in the Republic of Korea since 2005, reduction in the incidence rate of varicella is not obvious. The study aimed to evaluate the vaccine effectiveness (VE) of one-dose UVV by timeline and severity of the disease.
Methods
All children born in Korea in 2011 were included for this retrospective cohort study that analyzed insurance claims data from 2011–2018 and the varicella vaccination records in the immunization registry. Adjusted hazard ratios by Cox proportional hazard models were used to estimate the VE through propensity score matching by the month of birth, sex, healthcare utilization rate, and region.
Results
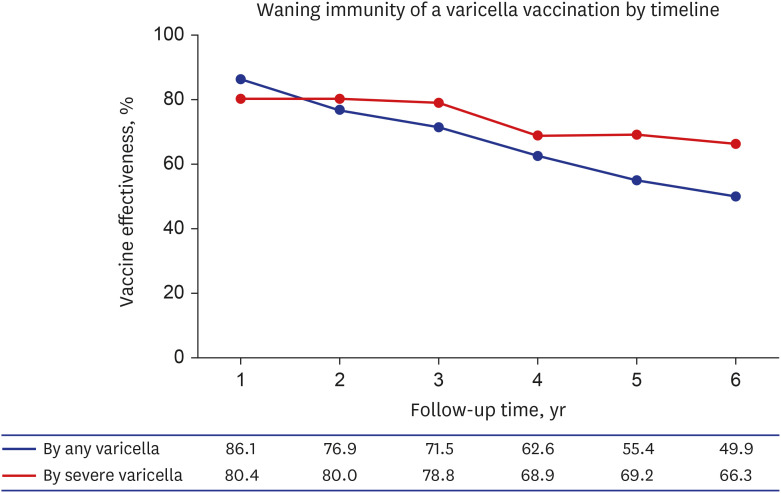
Of the total 421,070 newborns in the 2011 birth cohort, 13,360 were matched for age, sex, healthcare utilization rate, and region by the propensity score matching method. A total of 55,940 (13.29%) children were diagnosed with varicella, with the incidence rate 24.2 per 1000 person-year; 13.4% of vaccinated children and 10.4% of unvaccinated children. The VE of one-dose UVV against any varicella was 86.1% (95% confidence interval [CI], 81.4–89.5) during the first year after vaccination and 49.9% (95% CI, 43.3–55.7) during the 6-year followup period since vaccination, resulting in a 7.2% annual decrease of VE. The overall VE for severe varicella was 66.3%. The VE of two-dose compared to one-dose was 73.4% (95% CI, 72.2–74.6).
Conclusion
We found lower long-term VE in one-dose vaccination and waning of effectiveness over time. Longer follow ups of the vaccinated children as well as appropriately designed studies are needed to establish the optimal strategy in preventing varicella in Korea.
Keyword
Figure
Cited by 1 articles
-
Letter to the Editor: Effectiveness of the Varicella Vaccine Among Korean Children: Suggestions for Future Research
BongKyoo Choi, Hyunjeong Cho, Younchul Shin, Eun-Kyoung Lee
J Korean Med Sci. 2021;37(1):e17. doi: 10.3346/jkms.2022.37.e17.
Reference
-
1. Gilden DH, Kleinschmidt-DeMasters BK, LaGuardia JJ, Mahalingam R, Cohrs RJ. Neurologic complications of the reactivation of varicella-zoster virus. N Engl J Med. 2000; 342(9):635–645. PMID: 10699164.
Article2. Gnann JW Jr. Varicella-zoster virus: atypical presentations and unusual complications. J Infect Dis. 2002; 186(Suppl 1):S91–S98. PMID: 12353193.
Article3. Liese JG, Grote V, Rosenfeld E, Fischer R, Belohradsky BH, Kries R, et al. The burden of varicella complications before the introduction of routine varicella vaccination in Germany. Pediatr Infect Dis J. 2008; 27(2):119–124. PMID: 18227713.
Article4. Sheridan SL, Quinn HE, Hull BP, Ware RS, Grimwood K, Lambert SB. Impact and effectiveness of childhood varicella vaccine program in Queensland, Australia. Vaccine. 2017; 35(27):3490–3497. PMID: 28528765.
Article5. Siedler A, Rieck T, Tolksdorf K. Strong additional effect of a second varicella vaccine dose in children in Germany, 2009–2014. J Pediatr. 2016; 173:202–206.e2. PMID: 26995703.6. Varela FH, Pinto LA, Scotta MC. Global impact of varicella vaccination programs. Hum Vaccin Immunother. 2019; 15(3):645–657. PMID: 30427766.
Article7. Wang Y, Zhang L, Sun X, Cao Y, Wang Z, Liu L, et al. Effectiveness and failure rate of the varicella vaccine in an outbreak in Jiangsu, China: a 1:2 matched case-control study. Hum Vaccin Immunother. 2020; 16(3):506–512. PMID: 31526231.
Article8. Xu Y, Liu S, Che X, Liu Y, Zhang X, Du J, et al. Seroepidemiology of varicella in Hangzhou, China in the vaccine era. Hum Vaccin Immunother. 2018; 14(10):2464–2471. PMID: 30019992.
Article9. Xu Y, Liu Y, Zhang X, Zhang X, Du J, Cai Y, et al. Epidemiology of varicella and effectiveness of varicella vaccine in Hangzhou, China, 2019. Hum Vaccin Immunother. 2021; 17(1):211–216. PMID: 32574100.
Article10. Quinn HE, Gidding HF, Marshall HS, Booy R, Elliott EJ, Richmond P, et al. Varicella vaccine effectiveness over 10 years in Australia; moderate protection from 1-dose program. J Infect. 2019; 78(3):220–225. PMID: 30528868.
Article11. Sohn S, Hong K, Hwang H, Chun BC. Paradoxical health care utilization patterns among children in Korea who did not receive mandatory pneumococcal vaccination. Vaccine. 2021; 39(7):1096–1100. PMID: 33478789.
Article12. Chen TM, George S, Woodruff CA, Hsu S. Clinical manifestations of varicella-zoster virus infection. Dermatol Clin. 2002; 20(2):267–282. PMID: 12120440.
Article13. Gabutti G, Franchi M, Maniscalco L, Stefanati A. Varicella-zoster virus: pathogenesis, incidence patterns and vaccination programs. Minerva Pediatr. 2016; 68(3):213–225. PMID: 27125440.14. Choe YJ, Yang JJ, Park SK, Choi EH, Lee HJ. Comparative estimation of coverage between national immunization program vaccines and non-NIP vaccines in Korea. J Korean Med Sci. 2013; 28(9):1283–1288. PMID: 24015031.
Article15. Oh SH, Choi EH, Shin SH, Kim YK, Chang JK, Choi KM, et al. Varicella and varicella vaccination in South Korea. Clin Vaccine Immunol. 2014; 21(5):762–768. PMID: 24671555.
Article16. Lee YH, Choe YJ, Cho SI, Park H, Bang JH, Lee JK. Effects of one-dose varicella vaccination on disease severity in children during outbreaks in Seoul, Korea. J Korean Med Sci. 2019; 34(10):e83. PMID: 30886550.
Article17. Lee YH, Choe YJ, Cho SI, Bang JH, Oh MD, Lee JK. Increasing varicella incidence rates among children in the Republic of Korea: an age-period-cohort analysis. Epidemiol Infect. 2019; 147:e245. PMID: 31364576.
Article18. Choi JK, Park SH, Park S, Choi SM, Kim SH, Lee DG, et al. Trends in varicella and herpes zoster epidemiology before and after the implementation of universal one-dose varicella vaccination over one decade in South Korea, 2003–2015. Hum Vaccin Immunother. 2019; 15(11):2554–2560. PMID: 31008679.
Article19. Sohn S, Hong K, Chun BC. Evaluation of the effectiveness of pneumococcal conjugate vaccine for children in Korea with high vaccine coverage using a propensity score matched national population cohort. Int J Infect Dis. 2020; 93:146–150. PMID: 31982620.
Article20. Editors ICoMJ. Recommendations for the Conduct, Reporting, Editing and Publication of Scholarly Work in Medical Journals. 2019.21. Leung J, Broder KR, Marin M. Severe varicella in persons vaccinated with varicella vaccine (breakthrough varicella): a systematic literature review. Expert Rev Vaccines. 2017; 16(4):391–400. PMID: 28276305.
Article22. Arriola CS, Anderson EJ, Baumbach J, Bennett N, Bohm S, Hill M, et al. Does influenza vaccination modify influenza severity? Data on older adults hospitalized with influenza during the 2012–2013 season in the United States. J Infect Dis. 2015; 212(8):1200–1208. PMID: 25821227.
Article23. Caliendo M, Kopeinig S. Some practical guidance for the implementation of propensity score matching. J Econ Surv. 2008; 22(1):31–72.
Article24. Randolph JJ, Falbe K, Manuel AK, Balloun JL. A step-by-step guide to propensity score matching in R. PARE. 2014; 19(1):18.25. Fox J. Cox proportional-hazards regression for survival data. An R and S-PLUS companion to applied regression. Seattle, WA, USA: University of Washington;2002.26. Collett D. Modelling Survival Data in Medical Research. Boca Raton, FL, USA: CRC Press;2015.27. Zhu S, Zeng F, Xia L, He H, Zhang J. Incidence rate of breakthrough varicella observed in healthy children after 1 or 2 doses of varicella vaccine: Results from a meta-analysis. Am J Infect Control. 2018; 46(1):e1–e7. PMID: 28935482.
Article28. Huang WC, Huang LM, Chang IS, Tsai FY, Chang LY. Varicella breakthrough infection and vaccine effectiveness in Taiwan. Vaccine. 2011; 29(15):2756–2760. PMID: 21315697.
Article29. Asano Y, Suga S, Yoshikawa T, Kobayashi I, Yazaki T, Shibata M, et al. Experience and reason: twenty-year follow-up of protective immunity of the Oka strain live varicella vaccine. Pediatrics. 1994; 94(4 Pt 1):524–526. PMID: 7936864.
Article30. Di Pietrantonj C, Rivetti A, Marchione P, Debalini MG, Demicheli V. Vaccines for measles, mumps, rubella, and varicella in children. Cochrane Database Syst Rev. 2020; 4(4):CD004407. PMID: 32309885.
Article31. Dinleyici EC, Kurugol Z, Kara A, Tezer H, Tas MA, Guler E, et al. Children with breakthrough varicella infection requiring hospitalization in Turkey (VARICOMP Study 2008–2013). Vaccine. 2015; 33(32):3983–3987. PMID: 26133048.
Article32. Andrade AL, da Silva Vieira MA, Minamisava R, Toscano CM, de Lima Souza MB, Fiaccadori F, et al. Single-dose varicella vaccine effectiveness in Brazil: a case-control study. Vaccine. 2018; 36(4):479–483. PMID: 29249544.
Article33. Chan YD, Edmunds WJ, Chan HL, Wong ML, Au KA, Chuang SK, et al. Varicella vaccine dose depended effectiveness and waning among preschool children in Hong Kong. Hum Vaccin Immunother. 2020; 16(3):499–505. PMID: 31642729.
Article34. Lopez AS, Guris D, Zimmerman L, Gladden L, Moore T, Haselow DT, et al. One dose of varicella vaccine does not prevent school outbreaks: is it time for a second dose? Pediatrics. 2006; 117(6):e1070–e1077. PMID: 16740809.
Article35. Bonanni P, Gershon A, Gershon M, Kulcsár A, Papaevangelou V, Rentier B, et al. Primary versus secondary failure after varicella vaccination: implications for interval between 2 doses. Pediatr Infect Dis J. 2013; 32(7):e305–e313. PMID: 23838789.36. Rentier B, Gershon AA. European Working Group on Varicella. Consensus: varicella vaccination of healthy children--a challenge for Europe. Pediatr Infect Dis J. 2004; 23(5):379–389. PMID: 15131459.37. Gershon AA. Live-attenuated varicella vaccine. Infect Dis Clin North Am. 2001; 15(1):65–81. PMID: 11301823.
Article38. Vázquez M, LaRussa PS, Gershon AA, Niccolai LM, Muehlenbein CE, Steinberg SP, et al. Effectiveness over time of varicella vaccine. JAMA. 2004; 291(7):851–855. PMID: 14970064.
Article39. Liese JG, Cohen C, Rack A, Pirzer K, Eber S, Blum M, et al. The effectiveness of varicella vaccination in children in Germany: a case-control study. Pediatr Infect Dis J. 2013; 32(9):998–1004. PMID: 23694831.40. Barrenechea GG, Bastos LS. Evaluation of impact of one dose varicella vaccine on the incidence of chickenpox in Argentina. Vaccine. 2020; 38(2):330–335. PMID: 31630938.
Article41. Marin M, Marti M, Kambhampati A, Jeram SM, Seward JF. Global varicella vaccine effectiveness: a meta-analysis. Pediatrics. 2016; 137(3):e20153741. PMID: 26908671.
Article42. Pan X, Shu M, Ma R, Fang T, Dong H, Sun Y, et al. Varicella breakthrough infection and effectiveness of 2-dose varicella vaccine in China. Vaccine. 2018; 36(37):5665–5670. PMID: 30104113.
Article43. Leung J, Lopez AS, Blostein J, Thayer N, Zipprich J, Clayton A, et al. Impact of the US two-dose varicella vaccination program on the epidemiology of varicella outbreaks: data from nine states, 2005–2012. Pediatr Infect Dis J. 2015; 34(10):1105–1109. PMID: 26186103.44. Yin M, Xu X, Liang Y, Ni J. Effectiveness, immunogenicity and safety of one vs. two-dose varicella vaccination: a meta-analysis. Expert Rev Vaccines. 2018; 17(4):351–362. PMID: 29388450.45. Perella D, Wang C, Civen R, Viner K, Kuguru K, Daskalaki I, et al. Varicella vaccine effectiveness in preventing community transmission in the 2-dose era. Pediatrics. 2016; 137(4):e20152802. PMID: 26977081.
Article46. Esmaeeli S, Yaghoubi M, Nojomi M. Cost-effectiveness of varicella vaccination program in Iran. Int J Prev Med. 2017; 8(1):103. PMID: 29291045.
Article47. Lee YH, Choe YJ, Cho SI, Kang CR, Bang JH, Oh MD, et al. Effectiveness of varicella vaccination program in preventing laboratory-confirmed cases in children in Seoul, Korea. J Korean Med Sci. 2016; 31(12):1897–1901. PMID: 27822926.
Article48. Kim SH, Lee HJ, Park SE, Oh SH, Lee SY, Choi EH. Seroprevalence rate after one dose of varicella vaccine in infants. J Infect. 2010; 61(1):66–72. PMID: 20380851.
Article49. Ozasa K. The effect of misclassification on evaluating the effectiveness of influenza vaccines. Vaccine. 2008; 26(50):6462–6465. PMID: 18573297.
Article50. Emch M, Ali M, Park JK, Yunus M, Sack DA, Clemens JD. Relationship between neighbourhood-level killed oral cholera vaccine coverage and protective efficacy: evidence for herd immunity. Int J Epidemiol. 2006; 35(4):1044–1050. PMID: 16723370.
Article51. Salmon DA, Dudley MZ, Glanz JM, Omer SB. Vaccine hesitancy: causes, consequences, and a call to action. Vaccine. 2015; 33(Suppl 4):D66–D71. PMID: 26615171.