Ann Surg Treat Res.
2021 Sep;101(3):187-196. 10.4174/astr.2021.101.3.187.
Beneficial effects of posttransplant dipeptidyl peptidase-4 inhibitor administration after pancreas transplantation to improve β cell function
- Affiliations
-
- 1Division of Kidney and Pancreas Transplantation, Department of Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 2Asan Diabetes Center, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- KMID: 2519849
- DOI: http://doi.org/10.4174/astr.2021.101.3.187
Abstract
- Purpose
Dipeptidyl peptidase-4 (DPP-4) inhibitors lower blood glucose levels and enhance the function of pancreatic βcells. Yet, it is unknown whether posttransplant administration of DPP4 inhibitors is beneficial for pancreas transplant recipients.
Methods
We thus retrospectively analyzed the records of 312 patients who underwent pancreas transplantation between 2000 and 2018 at Asan Medical Center (Seoul, Korea) and compared the metabolic and survival outcomes according to DPP-4 inhibitor treatment.
Results
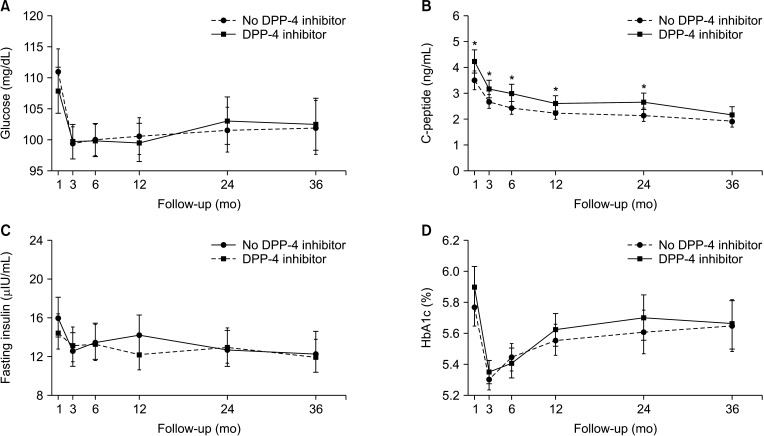
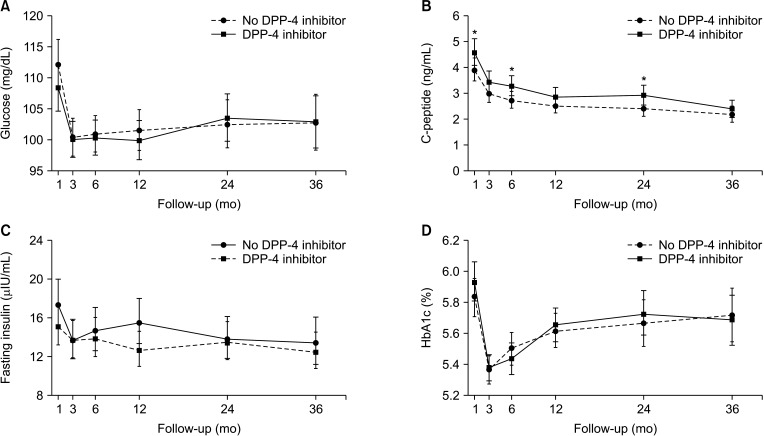
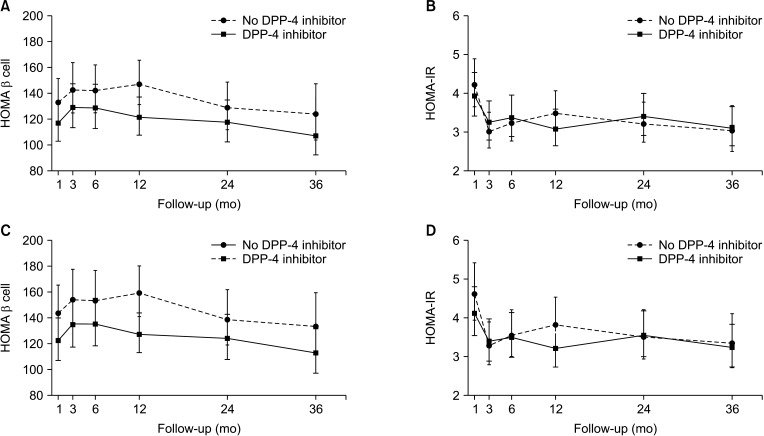
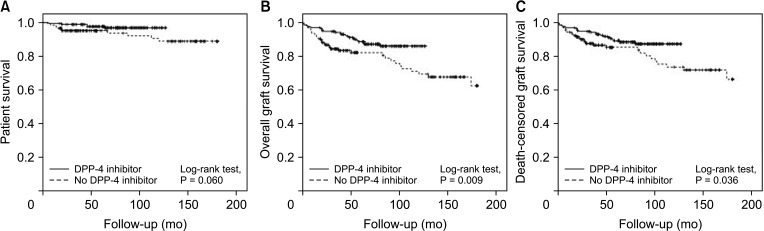
The patients were divided into the no DPP-4 inhibitor group (n = 165; no treatment with DPP-4 inhibitors or treated for <1 month) and the DPP-4 inhibitor group (n = 147; treated with DPP-4 inhibitors for ≥1 month). There were no significant differences in levels of glucose, hemoglobin A1c, and insulin between the 2 groups during 36 months of follow-up. However, the level of C-peptide was significantly higher in the DPP-4 inhibitor group at 1, 6, and 24 months posttransplant (all P < 0.05). Moreover, the DPP-4 inhibitor group had significantly higher rates of overall (log-rank test, P = 0.009) and death-censored (log-rank test, P = 0.036) graft survival during a 15-year follow-up.
Conclusion
Posttransplant DPP-4 inhibitor administration may help improve the clinical outcomes including β cell function after pancreas transplantation.
Keyword
Figure
Reference
-
1. Burke GW, Ciancio G, Sollinger HW. Advances in pancreas transplantation. Transplantation. 2004; 77(9 Suppl):S62–S67. PMID: 15201688.
Article2. Gillard P, Vandemeulebroucke E, Keymeulen B, Pirenne J, Maes B, De Pauw P, et al. Functional beta-cell mass and insulin sensitivity is decreased in insulin-independent pancreas-kidney recipients. Transplantation. 2009; 87:402–407. PMID: 19202446.3. Gruessner RW, Sutherland DE, Kandaswamy R, Gruessner AC. Over 500 solitary pancreas transplants in nonuremic patients with brittle diabetes mellitus. Transplantation. 2008; 85:42–47. PMID: 18192910.
Article4. Dean PG, Kukla A, Stegall MD, Kudva YC. Pancreas transplantation. BMJ. 2017; 357:j1321. PMID: 28373161.
Article5. Niederhaus SV, Kaufman DB, Odorico JS. Induction therapy in pancreas transplantation. Transpl Int. 2013; 26:704–714. PMID: 23672537.
Article6. Sutherland DE, Gruessner A. Longterm function (> 5 years) of pancreas grafts from the International Pancreas Transplant Registry database. Transplant Proc. 1995; 27:2977–2980. PMID: 8539798.7. Martin X, Feitosa Tajra LC, Benchaib M, Dawahra M, Lefrançois N, Dubernard JM. Long-term outcome of pancreas transplantation. Transplant Proc. 1997; 29:2423–2424. PMID: 9270792.
Article8. Sudan D, Sudan R, Stratta R. Longterm outcome of simultaneous kidney-pancreas transplantation: analysis of 61 patients with more than 5 years follow-up. Transplantation. 2000; 69:550–555. PMID: 10708110.9. Lo A, Stratta RJ, Hathaway DK, Egidi MF, Shokouh-Amiri MH, Grewal HP, et al. Long-term outcomes in simultaneous kidney-pancreas transplant recipients with portal-enteric versus systemicbladder drainage. Am J Kidney Dis. 2001; 38:132–143. PMID: 11431193.
Article10. Burke GW 3rd, Kaufman DB, Millis JM, Gaber AO, Johnson CP, Sutherland DE, et al. Prospective, randomized trial of the effect of antibody induction in simultaneous pancreas and kidney transplantation: three-year results. Transplantation. 2004; 77:1269–1275. PMID: 15114097.11. Farney AC, Doares W, Rogers J, Singh R, Hartmann E, Hart L, et al. A randomized trial of alemtuzumab versus antithymocyte globulin induction in renal and pancreas transplantation. Transplantation. 2009; 88:810–819. PMID: 19920781.
Article12. Stegall MD, Kim DY, Prieto M, Cohen AJ, Griffin MD, Schwab TR, et al. Thymoglobulin induction decreases rejection in solitary pancreas transplantation. Transplantation. 2001; 72:1671–1675. PMID: 11726830.
Article13. Katz H, Homan M, Velosa J, Robertson P, Rizza R. Effects of pancreas transplantation on postprandial glucose metabolism. N Engl J Med. 1991; 325:1278–1283. PMID: 1922222.
Article14. Robertson RP, Sutherland DE, Kendall DM, Teuscher AU, Gruessner RW, Gruessner A. Metabolic characterization of long-term successful pancreas transplants in type I diabetes. J Investig Med. 1996; 44:549–555.15. Shin S, Jung CH, Choi JY, Kwon HW, Jung JH, Kim YH, et al. Long-term metabolic outcomes of functioning pancreas transplants in type 2 diabetic recipients. Transplantation. 2017; 101:1254–1260. PMID: 27336397.
Article16. Dieterle CD, Arbogast H, Illner WD, Schmauss S, Landgraf R. Metabolic followup after long-term pancreas graft survival. Eur J Endocrinol. 2007; 156:603–610. PMID: 17468197.
Article17. Lauria MW, Figueiró JM, Machado LJ, Sanches MD, Nascimento GF, Lana AM, et al. Metabolic long-term follow-up of functioning simultaneous pancreas-kidney transplantation versus pancreas transplantation alone: insights and limitations. Transplantation. 2010; 89:83–87. PMID: 20061923.
Article18. Bae EJ. DPP-4 inhibitors in diabetic complications: role of DPP-4 beyond glucose cont rol. Arch Pharm Res. 2016; 39:1114–1128. PMID: 27502601.19. Stonehouse AH, Darsow T, Maggs DG. Incretin-based therapies. J Diabetes. 2012; 4:55–67. PMID: 21707956.
Article20. Singh B, Saxena A. Surrogate markers of insulin resistance: a review. World J Diabetes. 2010; 1:36–47. PMID: 21537426.
Article21. Shin S, Han DJ, Kim YH, Han S, Choi BH, Jung JH, et al. Long-term effects of delayed graft function on pancreas graft survival after pancreas transplantation. Transplantation. 2014; 98:1316–1322. PMID: 24839896.
Article22. Shin D, Eom YS, Chon S, Kim BJ, Yu KS, Lee DH. Factors influencing insulin sensitivity during hyperinsulinemic-euglycemic clamp in healthy Korean male subjects. Diabetes Metab Syndr Obes. 2019; 12:469–476. PMID: 31114276.23. Kang ES, Yun YS, Park SW, Kim HJ, Ahn CW, Song YD, et al. Limitation of the validity of the homeostasis model assessment as an index of insulin resistance in Korea. Metabolism. 2005; 54:206–211. PMID: 15690315.
Article24. Patil DT, Yerian LM. Pancreas transplant: recent advances and spectrum of features in pancreas allograft pathology. Adv Anat Pathol. 2010; 17:202–208. PMID: 20418674.25. Triñanes J, Ten Dijke P, Groen N, Hanegraaf M, Porrini E, Rodriguez-Rodriguez AE, et al. Tacrolimus-induced BMP/SMAD signaling associates with metabol ic stress-act ivated FOXO1 to trigger β-cel l fai lure. Diabetes. 2020; 69:193–204. PMID: 31732500.26. Shimizu S, Hosooka T, Matsuda T, Asahara S, Koyanagi-Kimura M, Kanno A, et al. DPP4 inhibitor vildagliptin preserves β-cell mass through amelioration of endoplasmic reticulum stress in C/EBPB transgenic mice. J Mol Endocrinol. 2012; 49:125–135. PMID: 22822047.
Article27. Galindo RJ, Fried M, Breen T, Tamler R. Hyperglycemia management in patients with posttransplantation diabetes. Endocr Pract. 2016; 22:454–465. PMID: 26720253.
Article28. Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006; 368:1696–1705. PMID: 17098089.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Counteracting the enzymatic activity of dipeptidylpeptidase 4 for potential therapeutic advantage, with an emphasis on cord blood transplantation
- Role of dipeptidyl peptidase-4 inhibitors in new-onset diabetes after transplantation
- Effect of Sodium-Glucose Cotransporter-2 Inhibitors versus Dipeptidyl Peptidase 4 Inhibitors on Cardiovascular Function in Patients with Type 2 Diabetes Mellitus and Coronary Artery Disease
- Triple Combination Therapy Using Metformin, Thiazolidinedione, and a GLP-1 Analog or DPP-IV Inhibitor in Patients with Type 2 Diabetes Mellitus