Endocrinol Metab.
2021 Aug;36(4):737-744. 10.3803/EnM.2021.401.
Exercise/Resistance Training and Muscle Stem Cells
- Affiliations
-
- 1Project for Muscle Stem Cell Biology, Graduate School of Pharmaceutical Sciences, Osaka University, Suita, Japan
- KMID: 2519661
- DOI: http://doi.org/10.3803/EnM.2021.401
Abstract
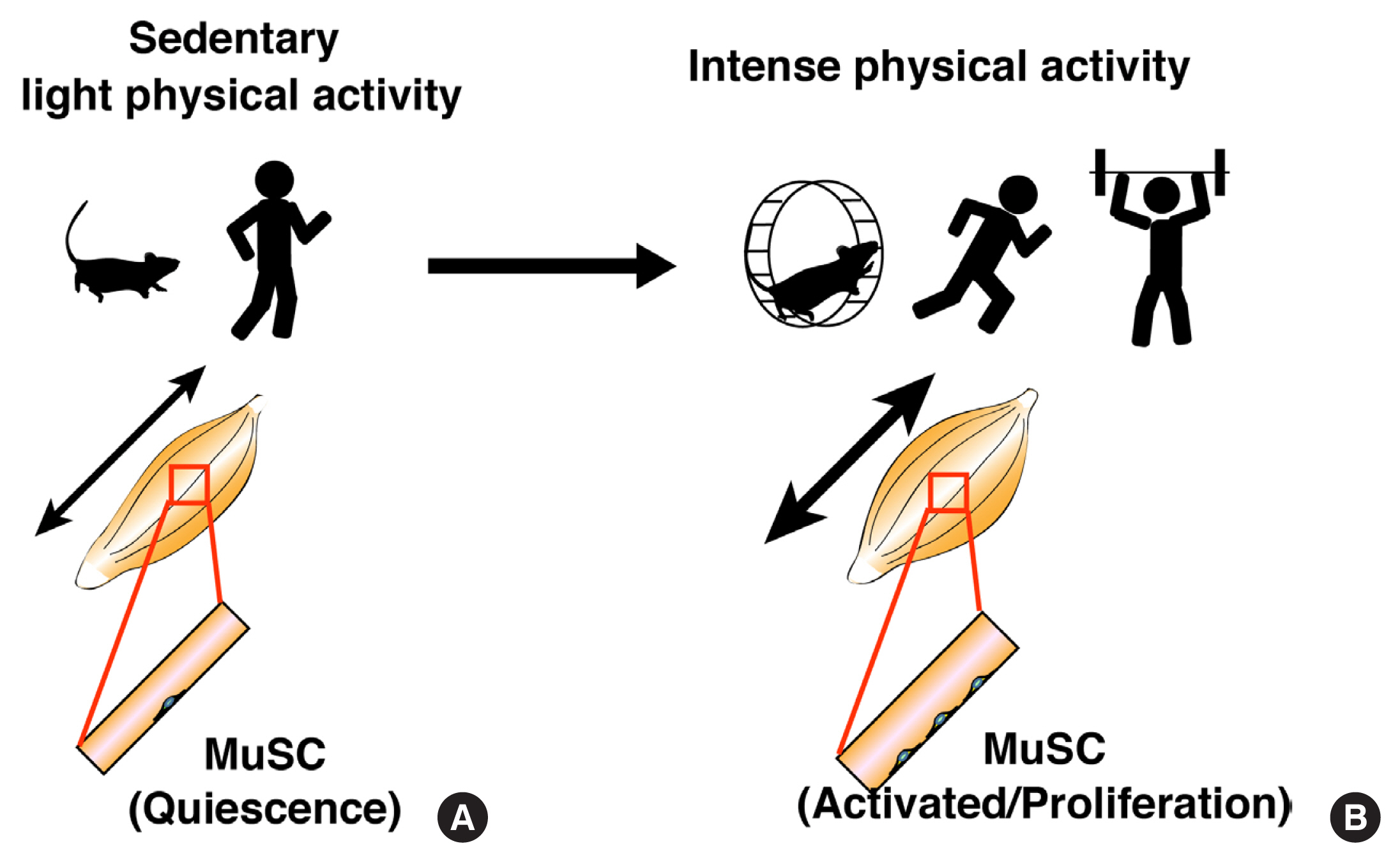
- Skeletal muscle has attracted attention as endocrine organ, because exercise-dependent cytokines called myokines/exerkines are released from skeletal muscle and are involved in systemic functions. While, local mechanical loading to skeletal muscle by exercise or resistance training alters myofiber type and size and myonuclear number. Skeletal muscle-resident stem cells, known as muscle satellite cells (MuSCs), are responsible for the increased number of myonuclei. Under steady conditions, MuSCs are maintained in a mitotically quiescent state but exit from that state and start to proliferate in response to high physical activity. Alterations in MuSC behavior occur when myofibers are damaged, but the lethal damage to myofibers does not seem to evoke mechanical loading-dependent MuSC activation and proliferation. Given that MuSCs proliferate without damage, it is unclear how the different behaviors of MuSCs are controlled by different physical activities. Recent studies demonstrated that myonuclear number reflects the size of myofibers; hence, it is crucial to know the properties of MuSCs and the mechanism of myonuclear accretion by MuSCs. In addition, the elucidation of mechanical load-dependent changes in muscle resident cells, including MuSCs, will be necessary for the discovery of new myokines/exerkines and understating skeletal muscle diseases.
Keyword
Figure
Reference
-
1. Caspersen CJ, Powell KE, Christenson GM. Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep. 1985; 100:126–31.2. Hartman JH, Smith LL, Gordon KL, Laranjeiro R, Driscoll M, Sherwood DR, et al. Swimming exercise and transient food deprivation in caenorhabditis elegans promote mitochondrial maintenance and protect against chemical-induced mitotoxicity. Sci Rep. 2018; 8:8359.
Article3. Cartee GD, Hepple RT, Bamman MM, Zierath JR. Exercise promotes healthy aging of skeletal muscle. Cell Metab. 2016; 23:1034–47.
Article4. Agudelo LZ, Femenia T, Orhan F, Porsmyr-Palmertz M, Goiny M, Martinez-Redondo V, et al. Skeletal muscle PGC-1α1 modulates kynurenine metabolism and mediates resilience to stress-induced depression. Cell. 2014; 159:33–45.
Article5. Pedersen L, Idorn M, Olofsson GH, Lauenborg B, Nookaew I, Hansen RH, et al. Voluntary running suppresses tumor growth through epinephrine- and IL-6-dependent NK cell mobilization and redistribution. Cell Metab. 2016; 23:554–62.
Article6. Aoi W, Naito Y, Takagi T, Tanimura Y, Takanami Y, Kawai Y, et al. A novel myokine, secreted protein acidic and rich in cysteine (SPARC), suppresses colon tumorigenesis via regular exercise. Gut. 2013; 62:882–9.
Article7. Vinel C, Lukjanenko L, Batut A, Deleruyelle S, Pradere JP, Le Gonidec S, et al. The exerkine apelin reverses age-associated sarcopenia. Nat Med. 2018; 24:1360–71.
Article8. Son JS, Zhao L, Chen Y, Chen K, Chae SA, de Avila JM, et al. Maternal exercise via exerkine apelin enhances brown adipogenesis and prevents metabolic dysfunction in offspring mice. Sci Adv. 2020; 6:eaaz0359.
Article9. Laurens C, Parmar A, Murphy E, Carper D, Lair B, Maes P, et al. Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. 2020; 5:e131870.
Article10. Reddy A, Bozi LHM, Yaghi OK, Mills EL, Xiao H, Nicholson HE, et al. pH-gated succinate secretion regulates muscle remodeling in response to exercise. Cell. 2020; 183:62–75.
Article11. Bamman MM, Roberts BM, Adams GR. Molecular regulation of exercise-induced muscle fiber hypertrophy. Cold Spring Harb Perspect Med. 2018; 8:a029751.
Article12. Evano B, Tajbakhsh S. Skeletal muscle stem cells in comfort and stress. NPJ Regen Med. 2018; 3:24.
Article13. Mauro A. Satellite cell of skeletal muscle fibers. J Biophys Biochem Cytol. 1961; 9:493–5.
Article14. Lepper C, Partridge TA, Fan CM. An absolute requirement for Pax7-positive satellite cells in acute injury-induced skeletal muscle regeneration. Development. 2011; 138:3639–46.
Article15. Sambasivan R, Yao R, Kissenpfennig A, Van Wittenberghe L, Paldi A, Gayraud-Morel B, et al. Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development. 2011; 138:3647–56.
Article16. Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000; 102:777–86.
Article17. Irintchev A, Zeschnigk M, Starzinski-Powitz A, Wernig A. Expression pattern of M-cadherin in normal, denervated, and regenerating mouse muscles. Dev Dyn. 1994; 199:326–37.
Article18. Rosen GD, Sanes JR, LaChance R, Cunningham JM, Roman J, Dean DC. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992; 69:1107–19.
Article19. Blanco-Bose WE, Yao CC, Kramer RH, Blau HM. Purification of mouse primary myoblasts based on alpha 7 integrin expression. Exp Cell Res. 2001; 265:212–20.20. Fukada S, Ma Y, Ohtani T, Watanabe Y, Murakami S, Yamaguchi M. Isolation, characterization, and molecular regulation of muscle stem cells. Front Physiol. 2013; 4:317.
Article21. Ikemoto-Uezumi M, Uezumi A, Zhang L, Zhou H, Hashimoto N, Okamura K, et al. Reduced expression of calcitonin receptor is closely associated with age-related loss of the muscle stem cell pool. JCSM Rapid Commun. 2019; 2:1–13.
Article22. Fukada S, Uezumi A, Ikemoto M, Masuda S, Segawa M, Tanimura N, et al. Molecular signature of quiescent satellite cells in adult skeletal muscle. Stem Cells. 2007; 25:2448–59.
Article23. Yamaguchi M, Ogawa R, Watanabe Y, Uezumi A, Miyagoe-Suzuki Y, Tsujikawa K, et al. Calcitonin receptor and Odz4 are differently expressed in Pax7-positive cells during skeletal muscle regeneration. J Mol Histol. 2012; 43:581–7.
Article24. Chakkalakal JV, Jones KM, Basson MA, Brack AS. The aged niche disrupts muscle stem cell quiescence. Nature. 2012; 490:355–60.
Article25. Ono Y, Masuda S, Nam HS, Benezra R, Miyagoe-Suzuki Y, Takeda S. Slow-dividing satellite cells retain long-term self-renewal ability in adult muscle. J Cell Sci. 2012; 125(Pt 5):1309–17.
Article26. Der Vartanian A, Quetin M, Michineau S, Aurade F, Hayashi S, Dubois C, et al. PAX3 confers functional heterogeneity in skeletal muscle stem cell responses to environmental stress. Cell Stem Cell. 2019; 24:958–73.
Article27. Rocheteau P, Gayraud-Morel B, Siegl-Cachedenier I, Blasco MA, Tajbakhsh S. A subpopulation of adult skeletal muscle stem cells retains all template DNA strands after cell division. Cell. 2012; 148:112–25.
Article28. Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007; 129:999–1010.
Article29. Scaramozza A, Park D, Kollu S, Beerman I, Sun X, Rossi DJ, et al. Lineage tracing reveals a subset of reserve muscle stem cells capable of clonal expansion under stress. Cell Stem Cell. 2019; 24:944–57.
Article30. Garcia-Prat L, Perdiguero E, Alonso-Martin S, Dell’Orso S, Ravichandran S, Brooks SR, et al. FoxO maintains a genuine muscle stem-cell quiescent state until geriatric age. Nat Cell Biol. 2020; 22:1307–18.
Article31. Motohashi N, Uezumi A, Asakura A, Ikemoto-Uezumi M, Mori S, Mizunoe Y, et al. Tbx1 regulates inherited metabolic and myogenic abilities of progenitor cells derived from slow- and fast-type muscle. Cell Death Differ. 2019; 26:1024–36.
Article32. Evano B, Gill D, Hernando-Herraez I, Comai G, Stubbs TM, Commere PH, et al. Transcriptome and epigenome diversity and plasticity of muscle stem cells following transplantation. PLoS Genet. 2020; 16:e1009022.
Article33. Li P, Akimoto T, Zhang M, Williams RS, Yan Z. Resident stem cells are not required for exercise-induced fiber-type switching and angiogenesis but are necessary for activity-dependent muscle growth. Am J Physiol Cell Physiol. 2006; 290:C1461–8.
Article34. Masschelein E, D’Hulst G, Zvick J, Hinte L, Soro-Arnaiz I, Gorski T, et al. Exercise promotes satellite cell contribution to myofibers in a load-dependent manner. Skelet Muscle. 2020; 10:21.
Article35. Yamamoto M, Legendre NP, Biswas AA, Lawton A, Yamamoto S, Tajbakhsh S, et al. Loss of MyoD and Myf5 in skeletal muscle stem cells results in altered myogenic programming and failed regeneration. Stem Cell Reports. 2018; 10:956–69.
Article36. Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010; 12:143–52.
Article37. Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010; 12:153–63.
Article38. Uezumi A, Ito T, Morikawa D, Shimizu N, Yoneda T, Segawa M, et al. Fibrosis and adipogenesis originate from a common mesenchymal progenitor in skeletal muscle. J Cell Sci. 2011; 124(Pt 21):3654–64.
Article39. McCarthy JJ, Mula J, Miyazaki M, Erfani R, Garrison K, Farooqui AB, et al. Effective fiber hypertrophy in satellite cell-depleted skeletal muscle. Development. 2011; 138:3657–66.
Article40. Englund DA, Murach KA, Dungan CM, Figueiredo VC, Vechetti IJ Jr, Dupont-Versteegden EE, et al. Depletion of resident muscle stem cells negatively impacts running volume, physical function, and muscle fiber hypertrophy in response to lifelong physical activity. Am J Physiol Cell Physiol. 2020; 318:C1178–88.
Article41. Fukada SI, Akimoto T, Sotiropoulos A. Role of damage and management in muscle hypertrophy: different behaviors of muscle stem cells in regeneration and hypertrophy. Biochim Biophys Acta Mol Cell Res. 2020; 1867:118742.
Article42. Fukuda S, Kaneshige A, Kaji T, Noguchi YT, Takemoto Y, Zhang L, et al. Sustained expression of HeyL is critical for the proliferation of muscle stem cells in overloaded muscle. Elife. 2019; 8:e48284.
Article43. Darr KC, Schultz E. Exercise-induced satellite cell activation in growing and mature skeletal muscle. J Appl Physiol (1985). 1987; 63:1816–21.
Article44. Fuchs E, Blau HM. Tissue stem cells: architects of their niches. Cell Stem Cell. 2020; 27:532–56.
Article45. Zhang L, Noguchi YT, Nakayama H, Kaji T, Tsujikawa K, Ikemoto-Uezumi M, et al. The CalcR-PKA-Yap1 axis is critical for maintaining quiescence in muscle stem cells. Cell Rep. 2019; 29:2154–63.
Article46. Yamaguchi M, Watanabe Y, Ohtani T, Uezumi A, Mikami N, Nakamura M, et al. Calcitonin receptor signaling inhibits muscle stem cells from escaping the quiescent state and the niche. Cell Rep. 2015; 13:302–14.
Article47. Bjornson CR, Cheung TH, Liu L, Tripathi PV, Steeper KM, Rando TA. Notch signaling is necessary to maintain quiescence in adult muscle stem cells. Stem Cells. 2012; 30:232–42.
Article48. Mourikis P, Sambasivan R, Castel D, Rocheteau P, Bizzarro V, Tajbakhsh S. A critical requirement for notch signaling in maintenance of the quiescent skeletal muscle stem cell state. Stem Cells. 2012; 30:243–52.
Article49. Noguchi YT, Nakamura M, Hino N, Nogami J, Tsuji S, Sato T, et al. Cell-autonomous and redundant roles of Hey1 and HeyL in muscle stem cells: HeyL requires Hes1 to bind diverse DNA sites. Development. 2019; 146:dev163618.
Article50. Lahmann I, Brohl D, Zyrianova T, Isomura A, Czajkowski MT, Kapoor V, et al. Oscillations of MyoD and Hes1 proteins regulate the maintenance of activated muscle stem cells. Genes Dev. 2019; 33:524–35.
Article51. Fukada S, Yamaguchi M, Kokubo H, Ogawa R, Uezumi A, Yoneda T, et al. Hesr1 and Hesr3 are essential to generate undifferentiated quiescent satellite cells and to maintain satellite cell numbers. Development. 2011; 138:4609–19.
Article52. Fujimaki S, Seko D, Kitajima Y, Yoshioka K, Tsuchiya Y, Masuda S, et al. Notch1 and notch2 coordinately regulate stem cell function in the quiescent and activated states of muscle satellite cells. Stem Cells. 2018; 36:278–85.
Article53. Mizuno S, Yoda M, Shimoda M, Tohmonda T, Okada Y, Toyama Y, et al. A disintegrin and metalloprotease 10 (ADAM10) is indispensable for maintenance of the muscle satellite cell pool. J Biol Chem. 2015; 290:28456–64.
Article54. Zammit PS, Golding JP, Nagata Y, Hudon V, Partridge TA, Beauchamp JR. Muscle satellite cells adopt divergent fates: a mechanism for self-renewal? J Cell Biol. 2004; 166:347–57.55. Lee JD, Fry CS, Mula J, Kirby TJ, Jackson JR, Liu F, et al. Aged muscle demonstrates fiber-type adaptations in response to mechanical overload, in the absence of myofiber hypertrophy, independent of satellite cell abundance. J Gerontol A Biol Sci Med Sci. 2016; 71:461–7.
Article56. Ikemoto-Uezumi M, Uezumi A, Tsuchida K, Fukada S, Yamamoto H, Yamamoto N, et al. Pro-insulin-like growth factor-II ameliorates age-related inefficient regenerative response by orchestrating self-reinforcement mechanism of muscle regeneration. Stem Cells. 2015; 33:2456–68.
Article57. Lukjanenko L, Karaz S, Stuelsatz P, Gurriaran-Rodriguez U, Michaud J, Dammone G, et al. Aging disrupts muscle stem cell function by impairing matricellular WISP1 secretion from fibro-adipogenic progenitors. Cell Stem Cell. 2019; 24:433–46.
Article58. Brett JO, Arjona M, Ikeda M, Quarta M, de Morree A, Egner IM, et al. Exercise rejuvenates quiescent skeletal muscle stem cells in old mice through restoration of cyclin D1. Nat Metab. 2020; 2:307–17.
Article59. Joanisse S, Nederveen JP, Baker JM, Snijders T, Iacono C, Parise G. Exercise conditioning in old mice improves skeletal muscle regeneration. FASEB J. 2016; 30:3256–68.
Article60. O’Connor RS, Pavlath GK. Point: counterpoint: satellite cell addition is/is not obligatory for skeletal muscle hypertrophy. J Appl Physiol (1985). 2007; 103:1099–100.61. McCarthy JJ, Dupont-Versteegden EE, Fry CS, Murach KA, Peterson CA. Methodological issues limit interpretation of negative effects of satellite cell depletion on adult muscle hypertrophy. Development. 2017; 144:1363–5.
Article62. Egner IM, Bruusgaard JC, Gundersen K. Satellite cell depletion prevents fiber hypertrophy in skeletal muscle. Development. 2016; 143:2898–906.
Article63. Fry CS, Lee JD, Jackson JR, Kirby TJ, Stasko SA, Liu H, et al. Regulation of the muscle fiber microenvironment by activated satellite cells during hypertrophy. FASEB J. 2014; 28:1654–65.64. Goh Q, Millay DP. Requirement of myomaker-mediated stem cell fusion for skeletal muscle hypertrophy. Elife. 2017; 6:e20007.
Article65. Goh Q, Song T, Petrany MJ, Cramer AA, Sun C, Sadayappan S, et al. Myonuclear accretion is a determinant of exercise-induced remodeling in skeletal muscle. Elife. 2019; 8:e44876.
Article66. Moriya N, Miyazaki M. Akt1 deficiency diminishes skeletal muscle hypertrophy by reducing satellite cell proliferation. Am J Physiol Regul Integr Comp Physiol. 2018; 314:R741–51.
Article67. Randrianarison-Huetz V, Papaefthymiou A, Herledan G, Noviello C, Faradova U, Collard L, et al. Srf controls satellite cell fusion through the maintenance of actin architecture. J Cell Biol. 2018; 217:685–700.
Article68. Cramer AAW, Prasad V, Eftestol E, Song T, Hansson KA, Dugdale HF, et al. Nuclear numbers in syncytial muscle fibers promote size but limit the development of larger myonuclear domains. Nat Commun. 2020; 11:6287.
Article69. Hansson KA, Eftestol E, Bruusgaard JC, Juvkam I, Cramer AW, Malthe-Sorenssen A, et al. Myonuclear content regulates cell size with similar scaling properties in mice and humans. Nat Commun. 2020; 11:6288.
Article70. White RB, Bierinx AS, Gnocchi VF, Zammit PS. Dynamics of muscle fibre growth during postnatal mouse development. BMC Dev Biol. 2010; 10:21.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Resistance Exercise Strategies for Sarcopenic Obesity in Elderly People
- Effect of Exercise on Glucose Metabolism
- Effect of Exercise on Cardiovascular Disease in Patients with Diabetes Mellitus (Aerobic vs. Resistance)
- Effect of Resistance Exercise Training on Mustn1 mRNA Expression in Rat Skeletal Muscle
- The Effects of Aerobic/Resistance Exercise on Body Fat Mass, Muscle Strength and Endothelial Function in Korean Type 2 Diabetes mellitus Patients