J Korean Med Sci.
2021 Aug;36(31):e227. 10.3346/jkms.2021.36.e227.
Pediatric and Parents' Attitudes Towards COVID-19 Vaccines and Intention to Vaccinate for Children
- Affiliations
-
- 1Department of Pediatrics, Pusan National University Hospital, Busan, Korea
- 2Deparetment of Pediatrics, Pusan National University Children's Hospital, Yangsan, Korea
- 3Department of Pediatrics, Pusan National University School of Medicine, Yangsan, Korea
- KMID: 2519204
- DOI: http://doi.org/10.3346/jkms.2021.36.e227
Abstract
- Background
Coronavirus disease 2019 (COVID-19) vaccination is necessary to reach herd immunity and essential for mitigating the spread of the pandemic. In May 2021, the US FDA and the EU have expanded the emergency use authorization for a COVID-19 vaccine to children aged 12 to 15. The aim of this study was to investigate parental acceptability of COVID-19 vaccination for their children, factors affecting their acceptability, and children's perceptions of COVID-19 vaccines in Republic of Korea.
Methods
We conducted a questionnaire survey at two tertiary hospitals from May 25, 2021 to June 3, 2021. Subjects were parents having children under 18 years and children aged 10–18 years.
Results
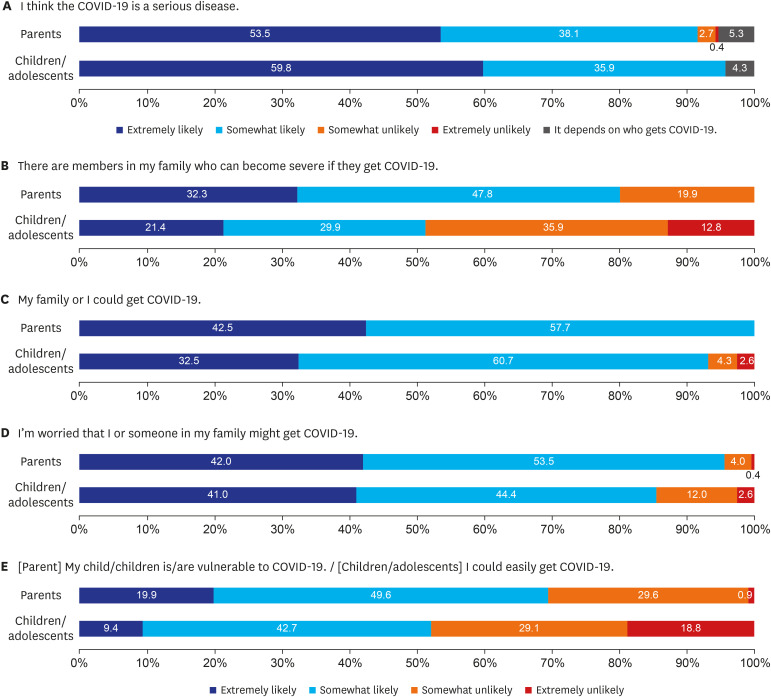
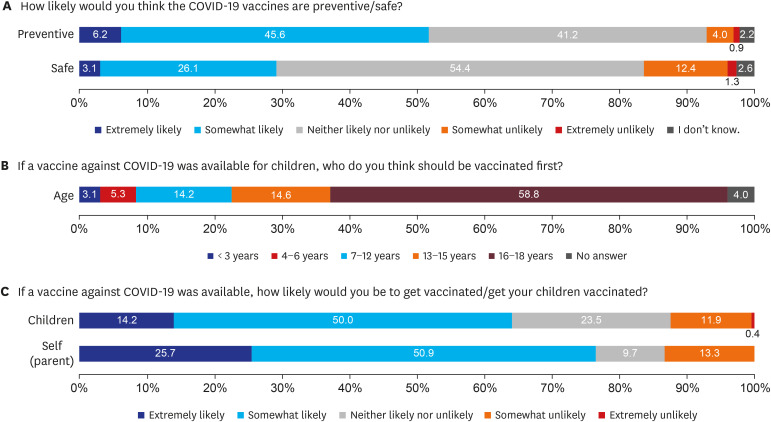
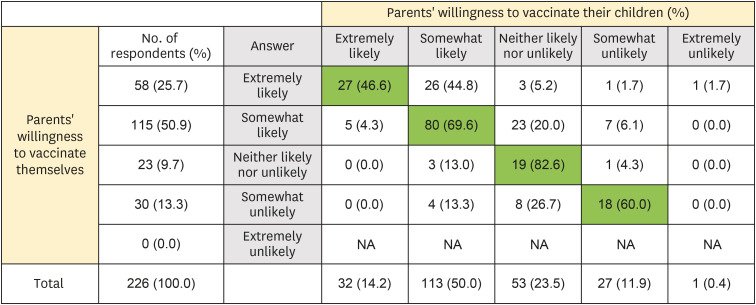
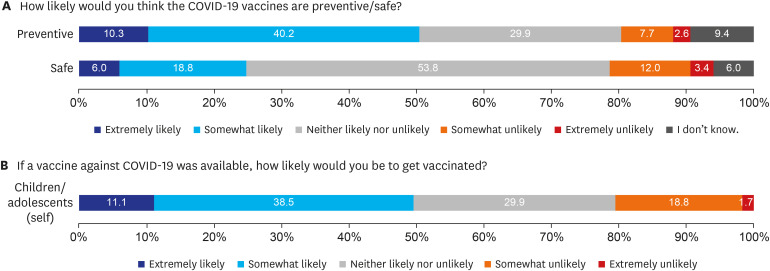
Two hundred twenty-six parents and 117 children aged 10–18 years were included in the final analysis. Overall, 76.5% and 64.2% of parents intended to get vaccinated against COVID-19 and intended to have their children vaccinated, respectively. However, only 49.6% of children responded that they would get COVID-19 vaccination. In the multivariate analysis, high confidence in the safety of COVID-19 vaccines (adjusted odds ratio [AOR], 4.87; 95% confidence interval [CI], 1.32–24.12), parents' willingness to vaccinate themselves (AOR, 19.42; 95% CI, 6.85–64.00), and awareness of the need to vaccinate children against COVID-19 (AOR, 13.15; 95% CI, 4.77–41.27) were associated with positive factors intention to vaccinate their children.
Conclusion
This study provides insight into how parents think about the COVID-19 vaccine for their children in South Korea. Our findings could be referenced in establishing a policy for childhood COVID-19 vaccination in the future.
Keyword
Figure
Cited by 2 articles
-
Expert Consensus on COVID-19 Vaccination in Korean Adolescents: A Modified Delphi Survey
Jae Hong Choi, Jihyun Moon, Seulgi Kim, Hyuna Bae, Jia Lee, Young June Choe
J Korean Med Sci. 2022;37(9):e69. doi: 10.3346/jkms.2022.37.e69.Effective Vaccination and Education Strategies for Emerging Infectious Diseases Such as COVID-19
Seong-Heon Wie, Jaehun Jung, Woo Joo Kim
J Korean Med Sci. 2023;38(44):e371. doi: 10.3346/jkms.2023.38.e371.
Reference
-
1. World Health Organization. WHO coronavirus (COVID-19) dashboard. Updated 2021. Accessed June 13, 2021. https://covid19.who.int/.2. Randolph HE, Barreiro LB. Herd immunity: understanding COVID-19. Immunity. 2020; 52(5):737–741. PMID: 32433946.
Article3. Chen X, Chen Z, Azman AS, Deng X, Sun R, Zhao Z, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Health. 2021; 9(5):e598–e609. PMID: 33705690.
Article4. Hossain A, Nasrullah SM, Tasnim Z, Hasan MK, Hasan MM. Seroprevalence of SARS-CoV-2 IgG antibodies among health care workers prior to vaccine administration in Europe, the USA and East Asia: a systematic review and meta-analysis. EClinicalMedicine. 2021; 33:100770. PMID: 33718853.
Article5. Rostami A, Sepidarkish M, Leeflang MM, Riahi SM, Nourollahpour Shiadeh M, Esfandyari S, et al. SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis. Clin Microbiol Infect. 2021; 27(3):331–340. PMID: 33228974.
Article6. World Health Organization. COVID-19 vaccine tracker and landscape. Updated 2021. Accessed June 13, 2021. https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines.7. World Health Organization. Emergency use listing procedure. COVID-19. Updated 2021. Accessed June 13, 2021. https://extranet.who.int/pqweb/vaccines/covid-19-vaccines.8. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes Pfizer-BioNTech COVID-19 vaccine for emergency use in adolescents in another important action in fight against pandemic. Updated 2021. Accessed June 13, 2021. https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-pfizer-biontech-covid-19-vaccine-emergency-use.9. European Centre for Disease Prevention and Control. Interim public health considerations for COVID-19 vaccination of adolescents in the EU/EEA. Updated 2021. Accessed June 13, 2021. https://www.ecdc.europa.eu/en/publications-data/interim-public-health-considerations-covid-19-vaccination-adolescents-eueea.10. Korea Disease Control and Prevention Agency. Press release, coronavirus disease-19, Republic of Korea. Updated 2021. Accessed June 13, 2021. http://ncov.mohw.go.kr/tcmBoardView.do?brdId=3&brdGubun=31&dataGubun=&ncvContSeq=5533&contSeq=5533&board_id=312&gubun=ALL.11. Kang HM, Choi EH, Kim YJ. Updates on the coronavirus disease 2019 vaccine and consideration in children. Clin Exp Pediatr. 2021; 64(7):328–338. PMID: 34148333.
Article12. Korea Disease Control and Prevention Agency. Press release, coronavirus disease-19, Republic of Korea. Updated 2021. Accessed June 10, 2021. http://ncov.mohw.go.kr/tcmBoardView.do?brdId=&brdGubun=&dataGubun=&ncvContSeq=365562&contSeq=365562&board_id=&gubun=ALL.13. Imperial College London. Global attitudes towards a COVID-19 vaccine. Updated 2021. Accessed June 12, 2021. https://www.imperial.ac.uk/media/imperial-college/institute-of-global-health-innovation/GlobalVaccineInsights_ICL-YouGov-Covid-19-Behaviour-Tracker_20210520_v2.pdf.14. Schwarzinger M, Watson V, Arwidson P, Alla F, Luchini S. COVID-19 vaccine hesitancy in a representative working-age population in France: a survey experiment based on vaccine characteristics. Lancet Public Health. 2021; 6(4):e210–e221. PMID: 33556325.
Article15. El-Elimat T, AbuAlSamen MM, Almomani BA, Al-Sawalha NA, Alali FQ. Acceptance and attitudes toward COVID-19 vaccines: a cross-sectional study from Jordan. PLoS One. 2021; 16(4):e0250555. PMID: 33891660.
Article16. Bell S, Clarke R, Mounier-Jack S, Walker JL, Paterson P. Parents' and guardians' views on the acceptability of a future COVID-19 vaccine: a multi-methods study in England. Vaccine. 2020; 38(49):7789–7798. PMID: 33109389.
Article17. Goldman RD, Marneni SR, Seiler M, Brown JC, Klein EJ, Cotanda CP, et al. Caregivers' willingness to accept expedited vaccine research during the COVID-19 pandemic: a cross-sectional survey. Clin Ther. 2020; 42(11):2124–2133. PMID: 33067013.
Article18. Goldman RD, Yan TD, Seiler M, Parra Cotanda C, Brown JC, Klein EJ, et al. Caregiver willingness to vaccinate their children against COVID-19: cross sectional survey. Vaccine. 2020; 38(48):7668–7673. PMID: 33071002.
Article19. Zhang KC, Fang Y, Cao H, Chen H, Hu T, Chen YQ, et al. Parental acceptability of COVID-19 vaccination for children under the age of 18 years: cross-sectional online survey. JMIR Pediatr Parent. 2020; 3(2):e24827. PMID: 33326406.
Article20. Brandstetter S, Böhmer MM, Pawellek M, Seelbach-Göbel B, Melter M, Kabesch M, et al. Parents' intention to get vaccinated and to have their child vaccinated against COVID-19: cross-sectional analyses using data from the KUNO-Kids health study. Eur J Pediatr. 2021.
Article21. The COVID States Project #45. Vaccine hesitancy and resistance among parents. Updated 2021. Accessed June 10, 2021. https://osf.io/e95bc/.22. Montalti M, Rallo F, Guaraldi F, Bartoli L, Po G, Stillo M, et al. Would parents get their children vaccinated against SARS-CoV-2? Rate and predictors of vaccine hesitancy according to a survey over 5000 families from Bologna, Italy. Vaccines (Basel). 2021; 9(4):366. PMID: 33920109.
Article23. ParentsTogether. Early research on parental attitudes about the COVID-19 vaccination & children. Updated 2021. Accessed June 12, 2021. https://parents-together.org/wp-content/uploads/2021/03/PT-Brief_-Parental-Attitudes-about-COVID-19-Vaccine.pdf.24. Yigit M, Ozkaya-Parlakay A, Senel E. Evaluation of COVID-19 vaccine refusal in parents. Pediatr Infect Dis J. 2021; 40(4):e134–e136. PMID: 33410650.
Article25. Centers for Disease Control and Prevention. What clinicians need to know about Pfizer-BioNTech COVID-19 vaccination of adolescents. Updated 2021. Accessed June 10, 2021. https://emergency.cdc.gov/coca/calls/2021/callinfo_051421.asp.26. Yılmaz M, Sahin MK. Parents' willingness and attitudes concerning the COVID-19 vaccine: a cross-sectional study. Int J Clin Pract. 2021.
Article27. Frenck RW Jr, Klein NP, Kitchin N, Gurtman A, Absalon J, Lockhart S, et al. Safety, immunogenicity, and efficacy of the BNT162b2 Covid-19 vaccine in adolescents. N Engl J Med. 2021; 385(3):239–250. PMID: 34043894.
Article28. Moderna. Phase 2/3 “TeenCOVE” study of mRNA-1273 in adolescents. Updated 2021. Accessed June 12, 2021. https://investors.modernatx.com/news-releases/news-release-details/moderna-reports-first-quarter-fiscal-year-2021-financial-results.29. Committee on Infectious Diseases. COVID-19 vaccines in children and adolescents. Pediatrics. 2021.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Examination of Predicting Factors for COVID-19 Vaccination Behaviors of University Students Utilizing the Theory of Planned Behavior
- Factors associated with parental intention to vaccinate their preschool children against COVID-19: a crosssectional survey in urban area of Jakarta, Indonesia
- Factors influencing COVID-19 vaccination intention among parents of children aged 5-11 years in South Korea: a cross-sectional study
- Parental concerns about COVID-19 vaccine safety and hesitancy in Korea: implications for vaccine communication
- Factors Affecting Parents’ Influenza Vaccination Intentions for Their Adolescent Children