Clin Exp Otorhinolaryngol.
2021 Aug;14(3):295-302. 10.21053/ceo.2020.01634.
Correlations Between the Adenotonsillar Microbiome and Clinical Characteristics of Pediatric Patients With Snoring
- Affiliations
-
- 1Department of Otorhinolaryngology-Head and Neck Surgery, Chung-Ang University College of Medicine, Seoul, Korea
- KMID: 2519184
- DOI: http://doi.org/10.21053/ceo.2020.01634
Abstract
Objectives
. Few studies have reported combined analyses of the microbiome of the adenoids and tonsils in pediatric patients with snoring, and correlations of the adenotonsillar microbiome with clinical characteristics have not been evaluated to date. The aim of this study was to characterize the adenotonsillar microbiome and to determine its correlations with the subjective symptoms of pediatric patients with snoring and with levels of regional mucosal immune molecules.
Methods
. Twenty-four children who underwent tonsillectomy with adenoidectomy owing to snoring were enrolled in this cross-sectional study conducted between August 2017 and December 2018. The microbiome of the adenoids and tonsils was characterized, and its alpha- and beta-diversity was determined. Clinical characteristics, including subjective discomfort during sleep (assessed using the obstructive sleep apnea-18 questionnaire), the presence of allergic rhinitis, concentrations of heat shock protein (Hsp)27, Hsp70, and interleukin-8 (IL-8) in lavage fluids, and white blood cell (WBC) counts, were measured.
Results
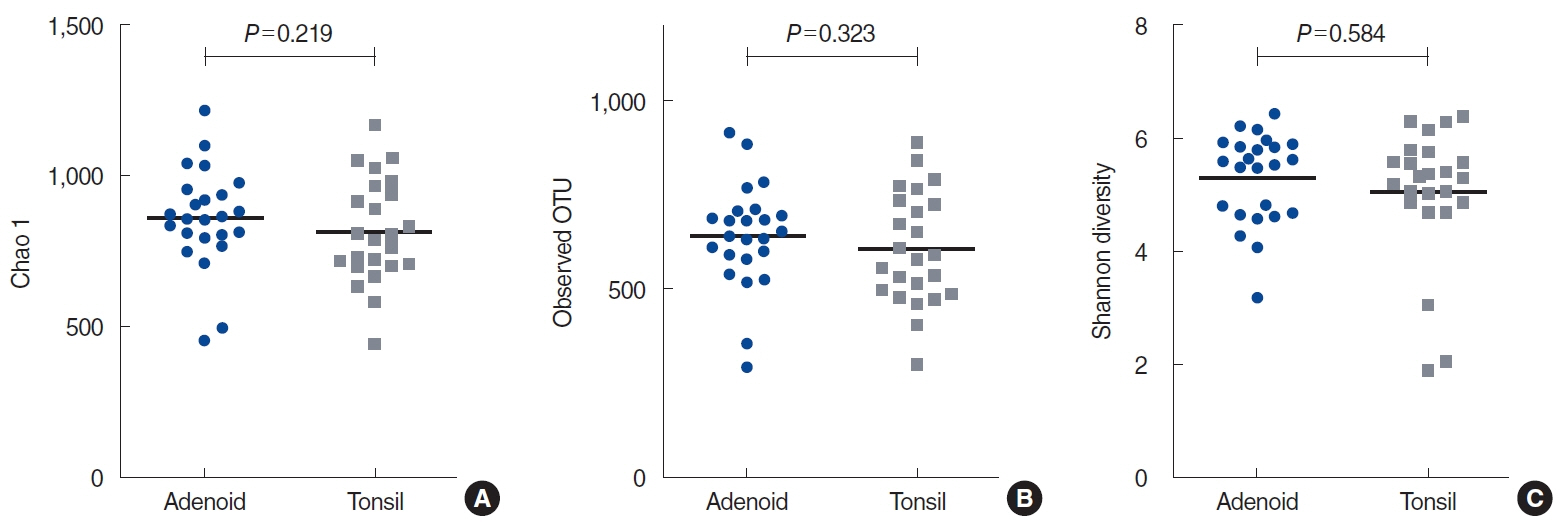
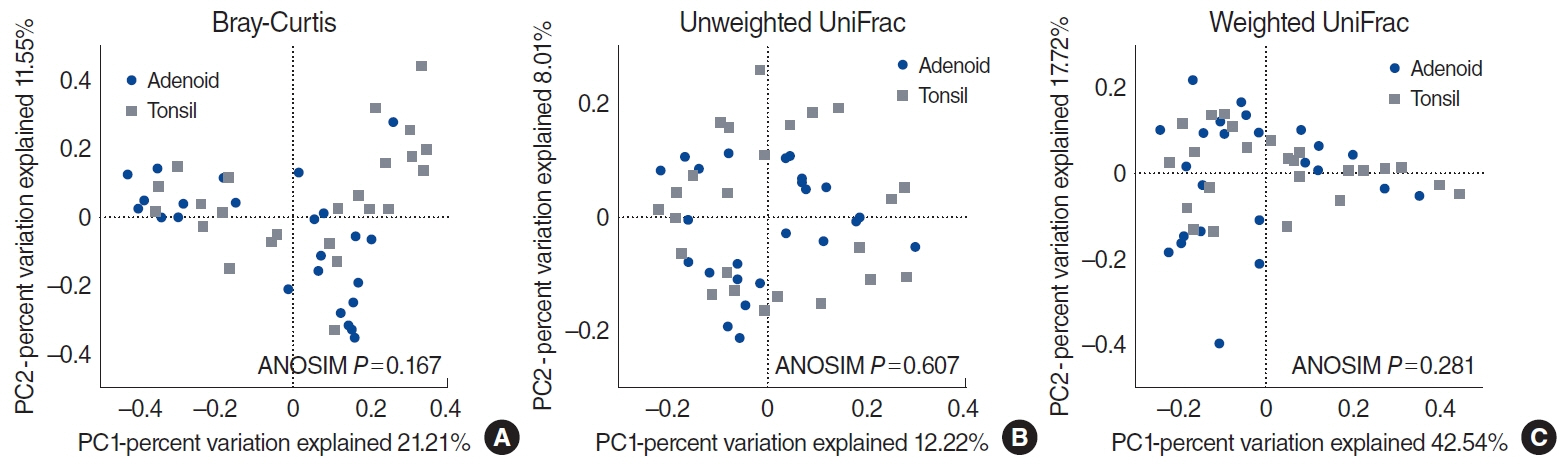
. At the phylum level, the microbiome was not significantly different between the adenoids and tonsils; the alpha and beta indices were likewise not significantly different between these two regions. The alpha-diversity of the entire adenotonsillar microbiome was associated with sex, emotional stress, and IL-8 levels in the tonsil lavage fluids. Beta-diversity was associated with Hsp27 levels in the tonsil lavage fluids and WBC counts. Multiple allergen simultaneous test results were not significant, although total serum immunoglobulin E levels were significantly associated with the beta-diversity of the adenotonsillar microbiome.
Conclusion
. The data reported herein suggest, for the first time, that the adenotonsillar microbiome interacts with the regional mucosal immune system. The observed association of the microbiome with subjective discomfort is a novel finding that warrants further investigation.
Keyword
Figure
Cited by 1 articles
-
What Does the Microbiome in the Tonsil Tell Us?
Sung-Woo Cho, Seung Koo Yang
Clin Exp Otorhinolaryngol. 2021;14(3):247-248. doi: 10.21053/ceo.2021.01074.
Reference
-
1. Gerhardsson H, Stalfors J, Odhagen E, Sunnergren O. Pediatric adenoid surgery in Sweden 2004-2013: incidence, indications and concomitant surgical procedures. Int J Pediatr Otorhinolaryngol. 2016; Aug. 87:61–6.
Article2. Juul ML, Rasmussen ER, Rasmussen SH, Sorensen CH, Howitz MF. A nationwide registry-based cohort study of incidence of tonsillectomy in Denmark, 1991-2012. Clin Otolaryngol. 2018; Feb. 43(1):274–84.
Article3. Zautner AE. Adenotonsillar disease. Recent Pat Inflamm Allergy Drug Discov. 2012; May. 6(2):121–9.4. Johnston J, Hoggard M, Biswas K, Astudillo-Garcia C, Radcliff FJ, Mahadevan M, et al. Paired analysis of the microbiota between surface tissue swabs and biopsies from pediatric patients undergoing adenotonsillectomy. Int J Pediatr Otorhinolaryngol. 2018; Oct. 113:51–7.
Article5. Xu Z, Wu Y, Tai J, Feng G, Ge W, Zheng L, et al. Risk factors of obstructive sleep apnea syndrome in children. J Otolaryngol Head Neck Surg. 2020; Mar. 49(1):11.
Article6. Johnston J, Hoggard M, Biswas K, Astudillo-Garcia C, Waldvogel-Thurlow S, Radcliff FJ, et al. The bacterial community and local lymphocyte response are markedly different in patients with recurrent tonsillitis compared to obstructive sleep apnoea. Int J Pediatr Otorhinolaryngol. 2018; Oct. 113:281–8.
Article7. Tsaoussoglou M, Lianou L, Maragozidis P, Hatzinikolaou S, Mavromati M, Orologas N, et al. Cysteinyl leukotriene receptors in tonsillar B- and T-lymphocytes from children with obstructive sleep apnea. Sleep Med. 2012; Aug. 13(7):879–85.
Article8. Fago-Olsen H, Dines LM, Sorensen CH, Jensen A. The adenoids but not the palatine tonsils serve as a reservoir for bacteria associated with secretory otitis media in small children. mSystems. 2019; Feb. 4(1):e00169–18.
Article9. Maw AR. Chronic otitis media with effusion and adeno-tonsillectomy: a prospective randomized controlled study. Int J Pediatr Otorhinolaryngol. 1983; Dec. 6(3):239–46.10. Min HJ, Park JS, Kim CE, Kim KS. Profiling of heat shock proteins 27 and 70 in adenoids of children. Eur Arch Otorhinolaryngol. 2019; Sep. 276(9):2483–9.
Article11. WHO Collaborating Center for Asthma and Rhinitis, Bousquet J, Anto JM, Demoly P, Schunemann HJ, Togias A, et al. Severe chronic allergic (and related) diseases: a uniform approach: a MeDALL-GA2LEN-ARIA position paper. Int Arch Allergy Immunol. 2012; 158(3):216–31.12. Kim JK, Yoon YM, Jang WJ, Choi YJ, Hong SC, Cho JH. Comparison study between MAST CLA and OPTIGEN. Am J Rhinol Allergy. 2011; Jul-Aug. 25(4):e156–9.
Article13. Hyun DW, Min HJ, Kim MS, Whon TW, Shin NR, Kim PS, et al. Dysbiosis of inferior turbinate microbiota is associated with high total IgE levels in patients with allergic rhinitis. Infect Immun. 2018; Mar. 86(4):e00934–17.
Article14. Ishman SL, Yang CJ, Cohen AP, Benke JR, Meinzen-Derr JK, Anderson RM, et al. Is the OSA-18 predictive of obstructive sleep apnea: comparison to polysomnography. Laryngoscope. 2015; Jun. 125(6):1491–5.
Article15. Hamady M, Walker JJ, Harris JK, Gold NJ, Knight R. Error-correcting barcoded primers for pyrosequencing hundreds of samples in multiplex. Nat Methods. 2008; Mar. 5(3):235–7.
Article16. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; May. 7(5):335–6.
Article17. Kim J, Bhattacharjee R, Dayyat E, Snow AB, Kheirandish-Gozal L, Goldman JL, et al. Increased cellular proliferation and inflammatory cytokines in tonsils derived from children with obstructive sleep apnea. Pediatr Res. 2009; Oct. 66(4):423–8.
Article18. Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011; Jun. 12(6):R60.
Article19. Wang X, Li H, Bezemer TM, Hao Z. Drivers of bacterial beta diversity in two temperate forests. Ecol Res. 2016; Jan. 31:57–64.
Article20. Vicente E, Marin JM, Carrizo SJ, Osuna CS, Gonzalez R, Marin-Oto M, et al. Upper airway and systemic inflammation in obstructive sleep apnoea. Eur Respir J. 2016; Oct. 48(4):1108–17.
Article21. De Boeck I, Wittouck S, Martens K, Claes J, Jorissen M, Steelant B, et al. Anterior nares diversity and pathobionts represent sinus microbiome in chronic rhinosinusitis. mSphere. 2019; Nov. 4(6):e00532–19.
Article22. Liu P, Wu L, Peng G, Han Y, Tang R, Ge J, et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav Immun. 2019; Aug. 80:633–43.
Article23. Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, et al. Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun. 2015; Aug. 48:186–94.
Article24. Perry M, Whyte A. Immunology of the tonsils. Immunol Today. 1998; Sep. 19(9):414–21.
Article25. Johnston JJ, Douglas R. Adenotonsillar microbiome: an update. Postgrad Med J. 2018; Jul. 94(1113):398–403.
Article26. Aktepe F, Sahin O, Dilek H, Yilmaz D, Kahveci O, Derekoy S. Immunohistochemical assesment of heat shock protein 70 in adenoid tissue. Int J Pediatr Otorhinolaryngol. 2007; Jun. 71(6):857–61.
Article27. Reddy VS, Madala SK, Trinath J, Reddy GB. Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones. 2018; May. 23(3):441–54.
Article28. Dogru M, Evcimik MF, Calim OF. Does adenoid hypertrophy affect disease severity in children with allergic rhinitis. Eur Arch Otorhinolaryngol. 2017; Jan. 274(1):209–213.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Effects of Adenotonsillectomy on Weight Change in Young Children
- Relationship Between Adenotonsillar Size and Snoring Sound: Acoustic Analysis
- Effect of adenotonsillar hypertrophy on snoring in children
- Clinical Implications of Snoring Time (%) in Patients with Obstructive Sleep Apnea
- The Urinary Microbiome: A Pediatric Urological Perspective