Ann Surg Treat Res.
2021 Aug;101(2):102-110. 10.4174/astr.2021.101.2.102.
Combined transabdominal and transperineal endoscopic pelvic exenteration for colorectal cancer: feasibility and safety of a two-team approach
- Affiliations
-
- 1Department of Surgical Oncology, Nagasaki University Graduate School of Biomedical Science, Nagasaki, Japan
- 2Department of Surgery, Sasebo City General Hospital, Nagasaki, Japan
- KMID: 2519003
- DOI: http://doi.org/10.4174/astr.2021.101.2.102
Abstract
- Purpose
Pelvic exenteration (PE) is a highly invasive procedure with high morbidity and mortality rates. Promising options to reduce this invasiveness have included laparoscopic and transperineal approaches. The aim of this study was to identify the safety of combined transabdominal and transperineal endoscopic PE for colorectal malignancies.
Methods
Fourteen patients who underwent combined transabdominal and transperineal PE (T group: 2-team approach, n = 7; O group: 1-team approach, n = 7) for colorectal malignancies between April 2016 and March 2020 in our institutions were included in this study. Clinicopathological features and perioperative outcomes were compared between groups.
Results
All patients successfully underwent R0 resection. Operation time tended to be shorter in the T group (463 minutes) than in the O group (636 minutes, P = 0.080). Time to specimen removal was significantly shorter (258 minutes vs. 423 minutes, P = 0.006), blood loss was lower (343 mL vs. 867 mL, P = 0.042), and volume of blood transfusion was less (0 mL vs. 560 mL, P = 0.063) in the T group, respectively. Postoperative complications were similar between groups.
Conclusion
Combined transabdominal and transperineal PE under a synchronous 2-team approach was feasible and safe, with the potential to reduce operation time, blood loss, and surgeon stress.
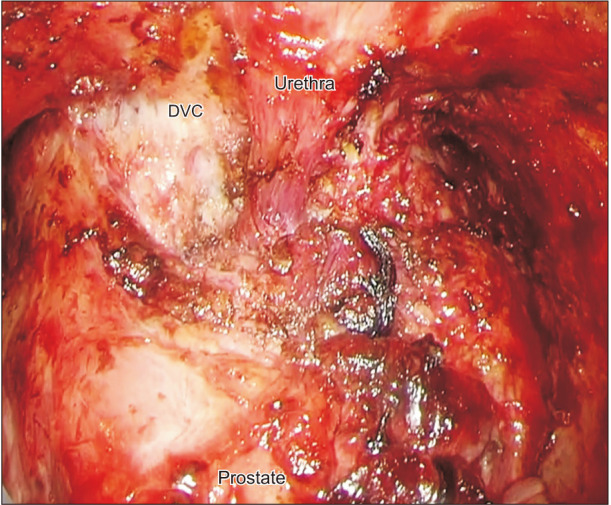
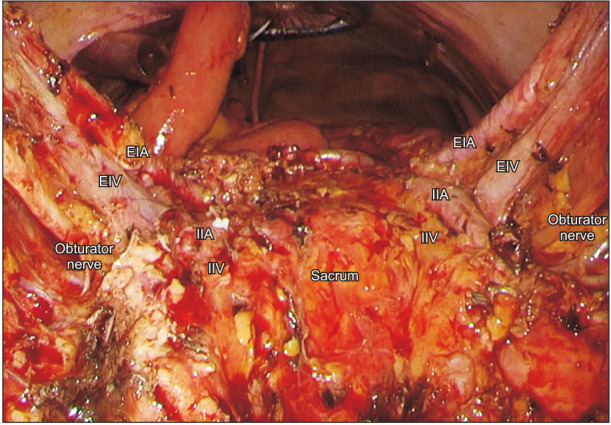
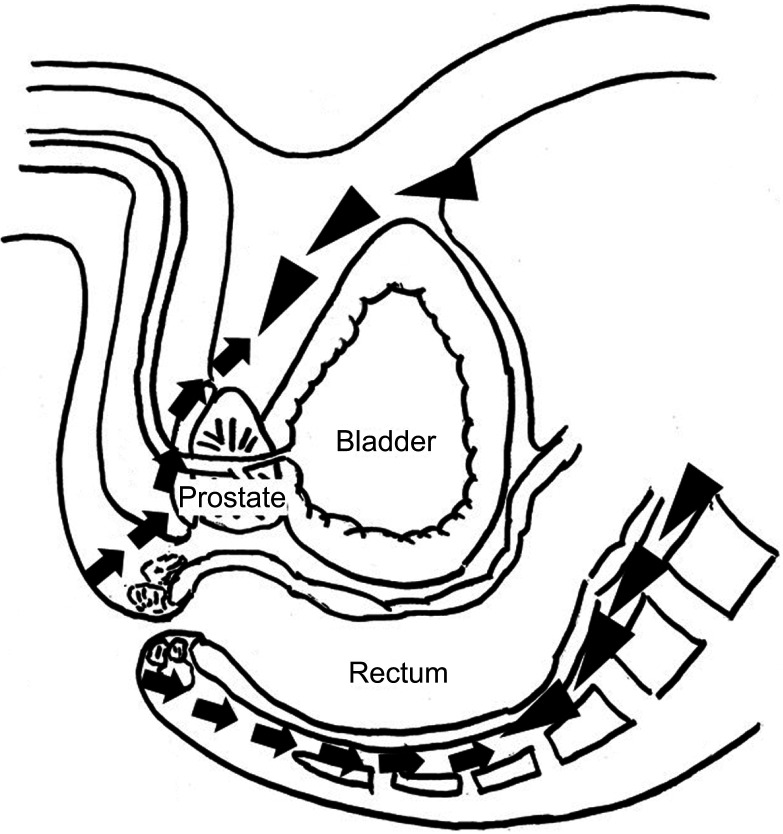
Figure
Reference
-
1. Smith JD, Nash GM, Weiser MR, Temple LK, Guillem JG, Paty PB. Multivisceral resections for rectal cancer. Br J Surg. 2012; 99:1137–1143. PMID: 22696063.
Article2. Quyn AJ, Austin KK, Young JM, Badgery-Parker T, Masya LM, Roberts R, et al. Outcomes of pelvic exenteration for locally advanced primary rectal cancer: overall survival and quality of life. Eur J Surg Oncol. 2016; 42:823–828. PMID: 26947962.
Article3. Pawlik TM, Skibber JM, Rodriguez-Bigas MA. Pelvic exenteration for advanced pelvic malignancies. Ann Surg Oncol. 2006; 13:612–623. PMID: 16538402.
Article4. Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma: a one-stage abdominoperineal operation with end colostomy and bilateral ureteral implantation into the colon above the colostomy. Cancer. 1948; 1:177–183. PMID: 18875031.5. Platt E, Dovell G, Smolarek S. Systematic review of outcomes following pelvic exenteration for the treatment of primary and recurrent locally advanced rectal cancer. Tech Coloproctol. 2018; 22:835–845. PMID: 30506497.
Article6. Yamamoto S, Inomata M, Katayama H, Mizusawa J, Etoh T, Konishi F, et al. Short-term surgical outcomes from a randomized controlled trial to evaluate laparoscopic and open D3 dissection for stage II/III colon cancer: Japan Clinical Oncology Group Study JCOG 0404. Ann Surg. 2014; 260:23–30. PMID: 24509190.7. Colon Cancer Laparoscopic or Open Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009; 10:44–52. PMID: 19071061.8. Peschaud F, Alves A, Berdah S, Kianmanesh R, Laurent C, Mabrut JY, et al. [Indications of laparoscopic general and digestive surgery. Evidence based guidelines of the French society of digestive surgery]. Ann Chir. 2006; 131:125–148. French. PMID: 16448622.9. Ogura A, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, Fukunaga Y, et al. Safety of laparoscopic pelvic exenteration with urinary diversion for colorectal malignancies. World J Surg. 2016; 40:1236–1243. PMID: 26643513.
Article10. Ichihara M, Uemura M, Ikeda M, Miyake M, Kato T, Hamakawa T, et al. Safety and feasibility of laparoscopic pelvic exenteration for locally advanced or recurrent colorectal cancer. Surg Laparosc Endosc Percutan Tech. 2019; 29:389–392. PMID: 31335481.
Article11. Uehara K, Nakamura H, Yoshino Y, Arimoto A, Kato T, Yokoyama Y, et al. Initial experience of laparoscopic pelvic exenteration and comparison with conventional open surgery. Surg Endosc. 2016; 30:132–138. PMID: 25795381.
Article12. de Lacy AM, Rattner DW, Adelsdorfer C, Tasende MM, Fernández M, Delgado S, et al. Transanal natural orifice transluminal endoscopic surgery (NOTES) rectal resection: “down-to-up” total mesorectal excision (TME): short-term outcomes in the first 20 cases. Surg Endosc. 2013; 27:3165–3172. PMID: 23519489.13. Atallah S, Martin-Perez B, Albert M, deBeche-Adams T, Nassif G, Hunter L, et al. Transanal minimally invasive surgery for total mesorectal excision (TAMISTME): results and experience with the first 20 patients undergoing curativeintent rectal cancer surgery at a single institution. Tech Coloproctol. 2014; 18:473–480. PMID: 24272607.
Article14. Arroyave MC, DeLacy FB, Lacy AM. Transanal total mesorectal excision (TaTME) for rectal cancer: step by step description of the surgical technique for a two- teams approach. Eur J Surg Oncol. 2017; 43:502–505. PMID: 27914773.15. Yamaguchi T, Imai M, Uematsu D. Hybrid approach using laparoscopy and transanal minimally invasive surgery to treat rectal cancer with invasion to the seminal vesicles. Asian J Endosc Surg. 2017; 10:219–222. PMID: 28547933.
Article16. Hasegawa S, Kajitani R, Matsumoto Y, Ohmiya T, Nagano H, Komono A, et al. Combined laparoscopic and transperineal endoscopic total pelvic exenteration for local recurrence of rectal cancer. Tech Coloproctol. 2020; 24:599–601. PMID: 32236744.
Article17. Harris DA, Davies M, Lucas MG, Drew P, Carr ND, Beynon J, et al. Multivisceral resection for primary locally advanced rectal carcinoma. Br J Surg. 2011; 98:582–588. PMID: 21656723.
Article18. PelvEx Collaborative. Surgical and survival outcomes following pelvic exenteration for locally advanced primary rectal cancer: results from an international collaboration. Ann Surg. 2019; 269:315–321. PMID: 28938268.19. Yang HY, Park SC, Hyun JH, Seo HK, Oh JH. Outcomes of pelvic exenteration for recurrent or primary locally advanced colorectal cancer. Ann Surg Treat Res. 2015; 89:131–137. PMID: 26366382.
Article20. Lei P, Ruan Y, Yang X, Fang J, Chen T. Trans-anal or trans-abdominal total mesorectal excision? A systematic review and meta-analysis of recent comparative studies on perioperative outcomes and pathological result. Int J Surg. 2018; 60:113–119. PMID: 30415089.
Article21. Akiyoshi T, Nagasaki T, Ueno M. Laparoscopic total pelvic exenteration for locally recurrent rectal cancer. Ann Surg Oncol. 2015; 22:3896. PMID: 25752892.
Article22. PelvEx Collaborative. Minimally invasive surgery techniques in pelvic exenteration: a systematic and meta-analysis review. Surg Endosc. 2018; 32:4707–4715. PMID: 30019221.23. Hasegawa S, Kajitani R, Munechika T, Matsumoto Y, Nagano H, Taketomi H, et al. Avoiding urethral and rectal injury during transperineal abdominoperineal resection in male patients with anorectal cancer. Surg Endosc. 2020; 34:4679–4682. PMID: 32430530.
Article24. Arora S, Sevdalis N, Nestel D, Woloshynowych M, Darzi A, Kneebone R. The impact of stress on surgical performance: a systematic review of the literature. Surgery. 2010; 147:318–330. 330.e1–330.e6. PMID: 20004924.
Article25. Pimentel G, Rodrigues S, Silva PA, Vilarinho A, Vaz R, Silva Cunha JP. A wearable approach for intraoperative physiological stress monitoring of multiple cooperative surgeons. Int J Med Inform. 2019; 129:60–68. PMID: 31445290.
Article26. Yang L, Money SR, Morrow MM, Lowndes BR, Weidner TK, Fortune E, et al. Impact of procedure type, case duration, and adjunctive equipment on surgeon intraoperative musculoskeletal discomfort. J Am Coll Surg. 2020; 230:554–560. PMID: 32220445.
Article27. Puntambekar S, Lawande A, Desai R, Puntambekar S, Joshi GA, Joshi SN. Initial experience of robotic anterior pelvic exenteration at a single institute. Int J Gynaecol Obstet. 2014; 126:41–44. PMID: 24786138.
Article28. Atallah S, Parra-Davila E, Melani AG. Assessment of the Versius surgical robotic system for dual-field synchronous transanal total mesorectal excision (taTME) in a preclinical model: will tomorrow's surgical robots promise newfound options? Tech Coloproctol. 2019; 23:471–477. PMID: 31069556.
Article29. Koedam TW, Veltcamp Helbach M, van de Ven PM, Kruyt PM, van Heek NT, Bonjer HJ, et al. Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol. 2018; 22:279–287. PMID: 29569099.
Article30. Lee L, Kelly J, Nassif GJ, deBeche-Adams TC, Albert MR, Monson JR. Defining the learning curve for transanal total mesorectal excision for rectal adenocarcinoma. Surg Endosc. 2020; 34:1534–1542. PMID: 29998391.
Article31. Larsen SG, Pfeffer F, Kørner H. Norwegian Colorectal Cancer Group. Norwegian moratorium on transanal total mesorectal excision. Br J Surg. 2019; 106:1120–1121. PMID: 31304578.
Article32. Francis N, Penna M, Mackenzie H, Carter F, Hompes R. International TaTME Educational Col laborative Group. Consensus on st ructured training curriculum for transanal total mesorectal excision (TaTME). Surg Endosc. 2017; 31:2711–2719. PMID: 28462478.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Combined laparoscopic and transperineal endoscopic total pelvic exenteration for the vaginal stump recurrence of cervical cancer
- Robotic pelvic exenteration and extended pelvic resections for locally advanced or synchronous rectal and urological malignancy
- Outcomes of pelvic exenteration for recurrent or primary locally advanced colorectal cancer
- Mesh-Based Transperineal Repair of a Perineal Hernia After a Laparoscopic Abdominoperineal Resection
- Minimally invasive surgery for maximally invasive tumors—pelvic exenterations for rectal cancers: are we prepared?