Diabetes Metab J.
2021 Jul;45(4):578-593. 10.4093/dmj.2020.0101.
Association of Combined TCF7L2 and KCNQ1 Gene Polymorphisms with Diabetic Micro- and Macrovascular Complications in Type 2 Diabetes Mellitus
- Affiliations
-
- 1Biomedical Sciences Program, Graduate School, Khon Kaen University, Khon Kaen, Thailand
- 2Cardiovascular Research Group, Khon Kaen University, Khon Kaen, Thailand
- 3Department of Medical Technology, School of Allied Health Sciences, Walailak University, Nakhon Si Thammarat, Thailand
- 4School of Medical Technology, Faculty of Associated Medical Science, Khon Kaen University, Khon Kaen, Thailand
- 5Department of Physiology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- 6Department of Medicine, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- 7Queen Sirikit Heart Center of the Northeast, Khon Kaen University, Khon Kaen, Thailand
- 8Department of Pathology, Faculty of Medicine, Khon Kaen University, Khon Kaen, Thailand
- KMID: 2518900
- DOI: http://doi.org/10.4093/dmj.2020.0101
Abstract
- Background
Vascular complications are the major morbid consequences of type 2 diabetes mellitus (T2DM). The transcription factor 7-like 2 (TCF7L2), potassium voltage-gated channel subfamily Q member 1 (KCNQ1), and inwardly-rectifying potassium channel, subfamily J, member 11 gene (KCNJ11) are common T2DM susceptibility genes in various populations. However, the associations between polymorphisms in these genes and diabetic complications are controversial. This study aimed to investigate the effects of combined gene-polymorphisms within TCF7L2, KCNQ1, and KCNJ11 on vascular complications in Thai subjects with T2DM.
Methods
We conducted a case-control study comprising 960 T2DM patients and 740 non-diabetes controls. Single nucleotide polymorphisms in TCF7L2, KCNQ1, and KCNJ11 were genotyped and evaluated for their association with diabetic vascular complications.
Results
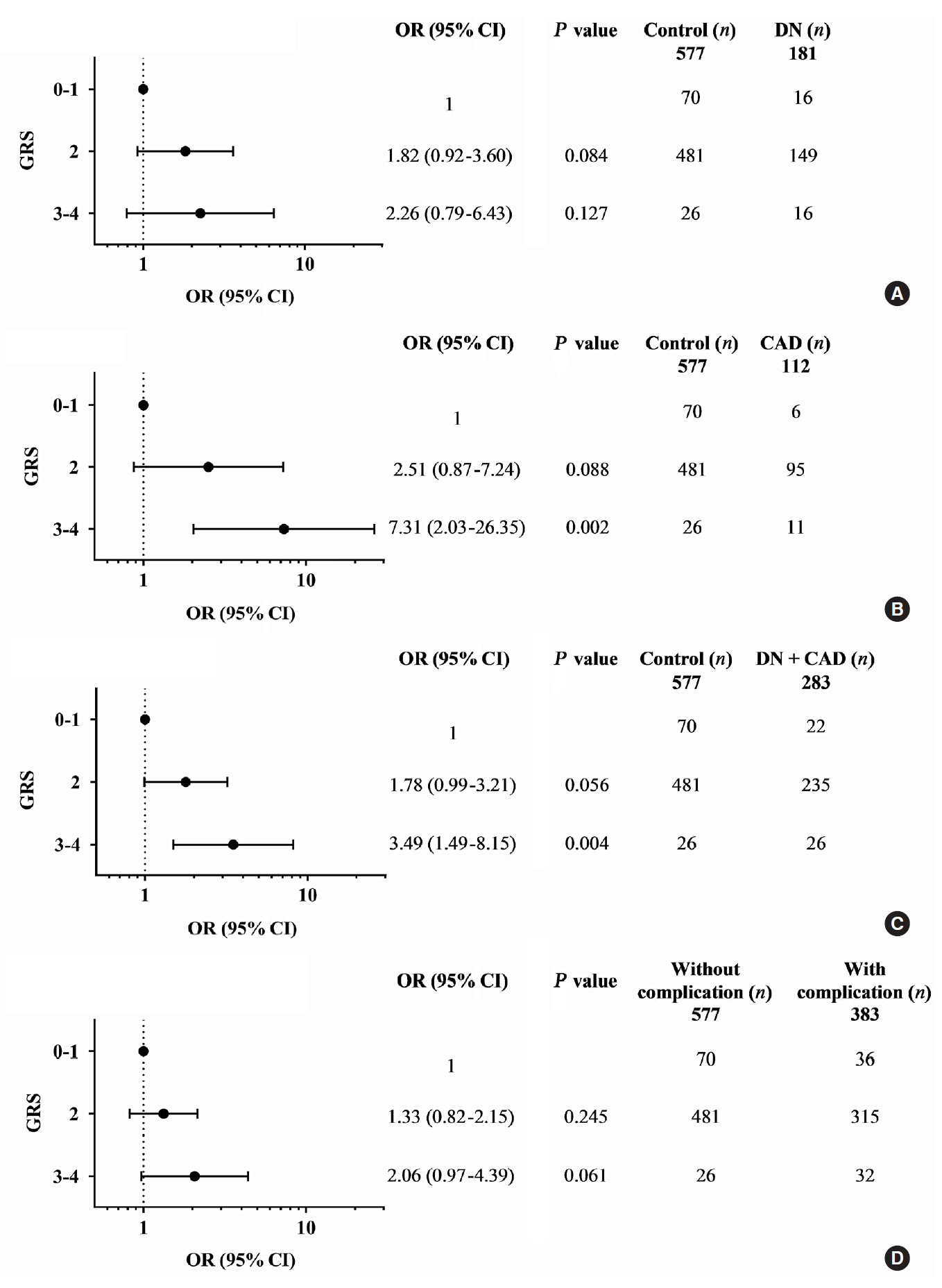
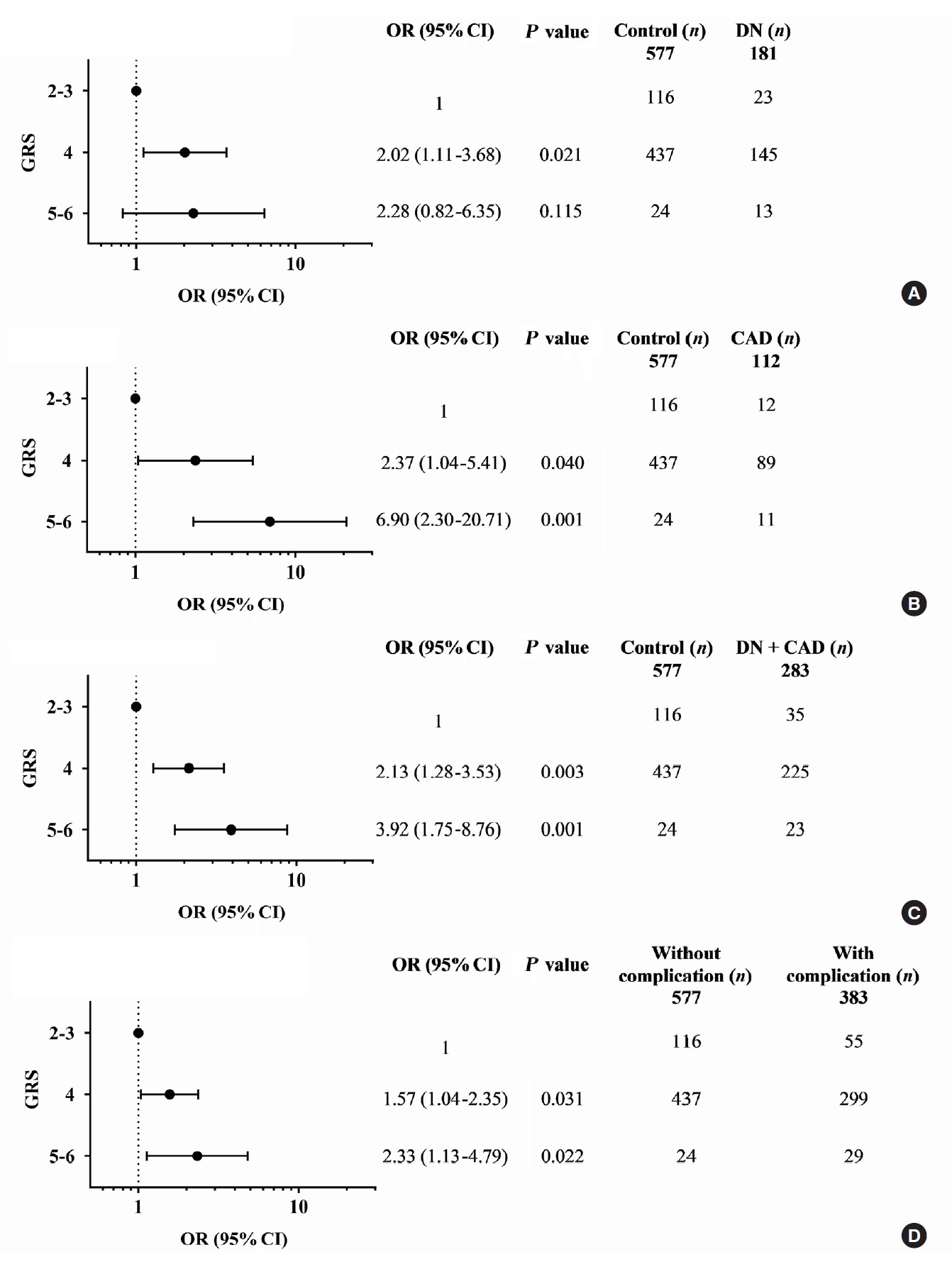
The gene variants TCF7L2 rs290487-T, KCNQ1 rs2237892-C, and KCNQ1 rs2237897-C were associated with increased risk of T2DM. TCF7L2 rs7903146-C, TCF7L2 rs290487-C, KCNQ1 rs2237892-T, and KCNQ1 rs2237897-T revealed an association with hypertension. The specific combination of risk-alleles that have effects on T2DM and hypertension, TCF7L2 rs7903146-C, KCNQ1 rs2237892-C, and KCNQ1 rs2237897-T, as genetic risk score (GRS), pronounced significant association with coronary artery disease (CAD), cumulative nephropathy and CAD, and cumulative microvascular and macrovascular complications (respective odds ratios [ORs] with 95% confidence interval [95% CI], comparing between GRS 2–3 and GRS 5–6, were 7.31 [2.03 to 26.35], 3.92 [1.75 to 8.76], and 2.33 [1.13 to 4.79]).
Conclusion
This study demonstrated, for the first time, the effect conferred by specific combined genetic variants in TCF7L2 and KCNQ1 on diabetic vascular complications, predominantly with nephropathy and CAD. Such a specific pattern of gene variant combination may implicate in the progression of T2DM and life-threatening vascular complications.
Keyword
Figure
Reference
-
1. Baena-Diez JM, Penafiel J, Subirana I, Ramos R, Elosua R, Marin-Ibanez A, et al. Risk of cause-specific death in individuals with diabetes: a competing risks analysis. Diabetes Care. 2016; 39:1987–95.2. Fowler MJ. Microvascular and macrovascular complications of diabetes. Clin Diabetes. 2008; 26:77–82.
Article3. Hurst C, Thinkhamrop B, Tran HT. The association between hypertension comorbidity and microvascular complications in type 2 diabetes patients: a nationwide cross-sectional study in Thailand. Diabetes Metab J. 2015; 39:395–404.
Article4. Cheung BM, Li C. Diabetes and hypertension: is there a common metabolic pathway? Curr Atheroscler Rep. 2012; 14:160–6.
Article5. Kosiborod M, Gomes MB, Nicolucci A, Pocock S, Rathmann W, Shestakova MV, et al. Vascular complications in patients with type 2 diabetes: prevalence and associated factors in 38 countries (the DISCOVER study program). Cardiovasc Diabetol. 2018; 17:150.
Article6. Sriwijitkamol A, Moungngern Y, Vannaseang S. Assessment and prevalences of diabetic complications in 722 Thai type 2 diabetes patients. J Med Assoc Thai. 2011; 94 Suppl 1:S168–74.7. Luo J, Zhao L, Chen AY, Zhang X, Zhu J, Zhao J, et al. TCF7L2 variation and proliferative diabetic retinopathy. Diabetes. 2013; 62:2613–7.
Article8. Ciccacci C, Di Fusco D, Cacciotti L, Morganti R, D’Amato C, Novelli G, et al. TCF7L2 gene polymorphisms and type 2 diabetes: association with diabetic retinopathy and cardiovascular autonomic neuropathy. Acta Diabetol. 2013; 50:789–99.
Article9. Buraczynska M, Zukowski P, Ksiazek P, Kuczmaszewska A, Janicka J, Zaluska W. Transcription factor 7-like 2 (TCF7L2) gene polymorphism and clinical phenotype in end-stage renal disease patients. Mol Biol Rep. 2014; 41:4063–8.
Article10. Zhuang Y, Niu F, Liu D, Sun J, Zhang X, Zhang J, et al. Associations of TCF7L2 gene polymorphisms with the risk of diabetic nephropathy: a case-control study. Medicine (Baltimore). 2018; 97:e8388.11. Zhang Y, Meng N, Lv Z, Li H, Qu Y. The gene polymorphisms of UCP1 but not PPAR γ and TCF7L2 are associated with diabetic retinopathy in Chinese type 2 diabetes mellitus cases. Acta Ophthalmol. 2015; 93:e223–9.12. Ohshige T, Tanaka Y, Araki S, Babazono T, Toyoda M, Umezono T, et al. A single nucleotide polymorphism in KCNQ1 is associated with susceptibility to diabetic nephropathy in Japanese subjects with type 2 diabetes. Diabetes Care. 2010; 33:842–6.
Article13. Zhang W, Wang H, Guan X, Niu Q, Li W. Variant rs2237892 of KCNQ1 is potentially associated with hypertension and macrovascular complications in type 2 diabetes mellitus in a Chinese Han population. Genomics Proteomics Bioinformatics. 2015; 13:364–70.
Article14. Al-Shammari MS, Al-Ali R, Al-Balawi N, Al-Enazi MS, Al-Muraikhi AA, Busaleh FN, et al. Type 2 diabetes associated variants of KCNQ1 strongly confer the risk of cardiovascular disease among the Saudi Arabian population. Genet Mol Biol. 2017; 40:586–90.
Article15. Riobello C, Gomez J, Gil-Pena H, Tranche S, Reguero JR, de la Hera JM, et al. KCNQ1 gene variants in the risk for type 2 diabetes and impaired renal function in the Spanish Renastur cohort. Mol Cell Endocrinol. 2016; 427:86–91.
Article16. Xiong C, Zheng F, Wan J, Zhou X, Yin Z, Sun X. The E23K polymorphism in Kir6.2 gene and coronary heart disease. Clin Chim Acta. 2006; 367:93–7.
Article17. Koo BK, Cho YM, Park BL, Cheong HS, Shin HD, Jang HC, et al. Polymorphisms of KCNJ11 (Kir6.2 gene) are associated with type 2 diabetes and hypertension in the Korean population. Diabet Med. 2007; 24:178–86.18. Liu NJ, Wu HH, Li YL, Yang Z, Tao XM, Du YP, et al. An analysis of the association between a polymorphism of KCNJ11 and diabetic retinopathy in a Chinese Han population. Eur J Med Res. 2015; 20:3.
Article19. Ip W, Chiang YT, Jin T. The involvement of the wnt signaling pathway and TCF7L2 in diabetes mellitus: the current understanding, dispute, and perspective. Cell Biosci. 2012; 2:28.
Article20. Priscakova P, Minarik G, Repiska V. Candidate gene studies of diabetic retinopathy in human. Mol Biol Rep. 2016; 43:1327–45.
Article21. Bonnet F, Roussel R, Natali A, Cauchi S, Petrie J, Laville M, et al. Parental history of type 2 diabetes, TCF7L2 variant and lower insulin secretion are associated with incident hypertension: data from the DESIR and RISC cohorts. Diabetologia. 2013; 56:2414–23.
Article22. Liin SI, Barro-Soria R, Larsson HP. The KCNQ1 channel: remarkable flexibility in gating allows for functional versatility. J Physiol. 2015; 593:2605–15.23. Ao D, Wang HJ, Wang LF, Song JY, Yang HX, Wang Y. The rs2237892 polymorphism in KCNQ1 influences gestational diabetes mellitus and glucose levels: a case-control study and meta-analysis. PLoS One. 2015; 10:e0128901.
Article24. van Vliet-Ostaptchouk JV, van Haeften TW, Landman GW, Reiling E, Kleefstra N, Bilo HJ, et al. Common variants in the type 2 diabetes KCNQ1 gene are associated with impairments in insulin secretion during hyperglycaemic glucose clamp. PLoS One. 2012; 7:e32148.
Article25. Ashcroft FM, Puljung MC, Vedovato N. Neonatal diabetes and the KATP channel: from mutation to therapy. Trends Endocrinol Metab. 2017; 28:377–87.26. Ashcroft FM, Rorsman P. Electrophysiology of the pancreatic beta-cell. Prog Biophys Mol Biol. 1989; 54:87–143.27. Chavali S, Mahajan A, Tabassum R, Dwivedi OP, Chauhan G, Ghosh S, et al. Association of variants in genes involved in pancreatic β-cell development and function with type 2 diabetes in North Indians. J Hum Genet. 2011; 56:695–700.
Article28. Yan J, Peng D, Jiang F, Zhang R, Chen M, Wang T, et al. Impaired pancreatic beta cell compensatory function is the main cause of type 2 diabetes in individuals with high genetic risk: a 9 year prospective cohort study in the Chinese population. Diabetologia. 2016; 59:1458–62.29. Marouli E, Kanoni S, Mamakou V, Hackinger S, Southam L, Prins B, et al. Evaluating the glucose raising effect of established loci via a genetic risk score. PLoS One. 2017; 12:e0186669.
Article30. Andersson EA, Allin KH, Sandholt CH, Borglykke A, Lau CJ, Ribel-Madsen R, et al. Genetic risk score of 46 type 2 diabetes risk variants associates with changes in plasma glucose and estimates of pancreatic β-cell function over 5 years of follow-up. Diabetes. 2013; 62:3610–7.
Article31. Stancakova A, Kuulasmaa T, Kuusisto J, Mohlke KL, Collins FS, Boehnke M, et al. Genetic risk scores in the prediction of plasma glucose, impaired insulin secretion, insulin resistance and incident type 2 diabetes in the METSIM study. Diabetologia. 2017; 60:1722–30.
Article32. American Diabetes Association. Standards of medical care in diabetes: 2013. Diabetes Care. 2013; 36 Suppl 1:S11–66.33. Petropoulos IN, Ponirakis G, Khan A, Almuhannadi H, Gad H, Malik RA. Diagnosing diabetic neuropathy: something old, something new. Diabetes Metab J. 2018; 42:255–69.
Article34. Tesfaye S, Boulton AJ, Dyck PJ, Freeman R, Horowitz M, Kempler P, et al. Diabetic neuropathies: update on definitions, diagnostic criteria, estimation of severity, and treatments. Diabetes Care. 2010; 33:2285–93.
Article35. Chaisakul J, Ukritchon S, Rangsin R, Mungthin M. Prevalence of peripheral neuropathy in Thai patients with type 2 diabetes and associated risk factors. J Med Assoc Thai. 2020; 103:254–61.36. Kaewput W, Thongprayoon C, Rangsin R, Mao MA, Satirapoj B, Cheungpasitporn W. The association between renal function and neurological diseases in type 2 diabetes: a multicenter nationwide cross-sectional study. Hosp Pract (1995). 2019; 47:46–52.
Article37. Weber MA, Schiffrin EL, White WB, Mann S, Lindholm LH, Kenerson JG, et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. J Clin Hypertens (Greenwich). 2014; 16:14–26.38. World Health Organization. The international association for the study of obesity, and the international obesity task force. Sydney: Health Communications Australia Pty Limited;2000. p. 56.39. Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 2001; 285:2486–97.40. Phani NM, Adhikari P, Nagri SK, D’Souza SC, Satyamoorthy K, Rai PS. Replication and relevance of multiple susceptibility loci discovered from genome wide association studies for type 2 diabetes in an Indian population. PLoS One. 2016; 11:e0157364.
Article41. Thomas N, Mahesh DM, Chapla A, Paul J, Shwetha N, Christina F, et al. Does TCF7L2 polymorphisms increase the risk of gestational diabetes mellitus in South Indian population? Endocr Abstr. 2014; 34:270.
Article42. Wang J, Hu F, Feng T, Zhao J, Yin L, Li L, et al. Meta-analysis of associations between TCF7L2 polymorphisms and risk of type 2 diabetes mellitus in the Chinese population. BMC Med Genet. 2013; 14:8.
Article43. Miyake K, Horikawa Y, Hara K, Yasuda K, Osawa H, Furuta H, et al. Association of TCF7L2 polymorphisms with susceptibility to type 2 diabetes in 4,087 Japanese subjects. J Hum Genet. 2008; 53:174–80.
Article44. Mussig K, Staiger H, Machicao F, Kirchhoff K, Guthoff M, Schafer SA, et al. Association of type 2 diabetes candidate polymorphisms in KCNQ1 with incretin and insulin secretion. Diabetes. 2009; 58:1715–20.
Article45. Unoki H, Takahashi A, Kawaguchi T, Hara K, Horikoshi M, Andersen G, et al. SNPs in KCNQ1 are associated with susceptibility to type 2 diabetes in East Asian and European populations. Nat Genet. 2008; 40:1098–102.
Article46. Rosengren AH, Braun M, Mahdi T, Andersson SA, Travers ME, Shigeto M, et al. Reduced insulin exocytosis in human pancreatic β-cells with gene variants linked to type 2 diabetes. Diabetes. 2012; 61:1726–33.
Article47. Torekov SS, Iepsen E, Christiansen M, Linneberg A, Pedersen O, Holst JJ, et al. KCNQ1 long QT syndrome patients have hyperinsulinemia and symptomatic hypoglycemia. Diabetes. 2014; 63:1315–25.
Article48. Fosmo AL, Skraastad OB. The Kv7 channel and cardiovascular risk factors. Front Cardiovasc Med. 2017; 4:75.
Article49. El-Atat FA, Stas SN, McFarlane SI, Sowers JR. The relationship between hyperinsulinemia, hypertension and progressive renal disease. J Am Soc Nephrol. 2004; 15:2816–27.
Article50. Sousa AG, Selvatici L, Krieger JE, Pereira AC. Association between genetics of diabetes, coronary artery disease, and macrovascular complications: exploring a common ground hypothesis. Rev Diabet Stud. 2011; 8:230–44.
Article51. Srivastava R, Zhang J, Go GW, Narayanan A, Nottoli TP, Mani A. Impaired LRP6-TCF7L2 activity enhances smooth muscle cell plasticity and causes coronary artery disease. Cell Rep. 2015; 13:746–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Epidemiology of Micro- and Macrovascular Complications of Type 2 Diabetes in Korea
- The Association of Diabetic Neuropathy with Other Chronic Diabetic Complications
- Non-Association between rs7903146 and rs12255372 Polymorphisms in Transcription Factor 7-Like 2 Gene and Type 2 Diabetes Mellitus in Jahrom City, Iran
- The association of chemokine receptor 5 gene 59029A/G polymorphisms with diabetic nephropathy in type 2 diabetic patients
- Risk Factors for Early Development of Macrovascular Complications in Korean Type 2 Diabetes