J Korean Med Sci.
2021 Jul;36(29):e191. 10.3346/jkms.2021.36.e191.
Should We Consider Value Frameworks for Cancer Drugs as Oncology's Landscape Evolves?; from an Oncologist Perspective in Korea
- Affiliations
-
- 1Department of Internal Medicine, Dongguk University Ilsan Hospital, Goyang, Korea
- 2Department of Internal Medicine, Inha University Hospital, Incheon, Korea
- 3Ewha Womans University, College of Pharmacy, Seoul, Korea
- 4Department of Internal Medicine, Seoul St. Mary's Hospital, The Catholic University of Korea, Seoul, Korea
- KMID: 2518738
- DOI: http://doi.org/10.3346/jkms.2021.36.e191
Abstract
- Background
As the role of immunotherapies and personalized medicine grow, cancer patients have faced many choices in treatments and have suffered financial toxicity. These challenges brought the need for the value framework (VF) to guide treatment decision making.
Methods
A survey was taken to 102 oncologists about perception for VF. They were asked about priorities among several considerations when they prescribe cancer drugs. Their views on the need for development and potential implications of VF in Korea were assessed, also.
Results
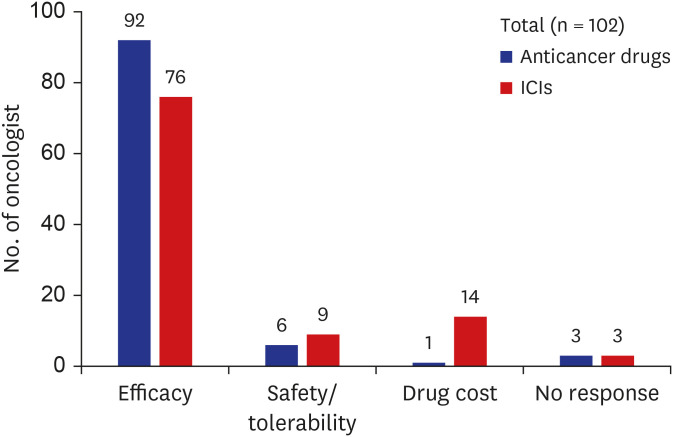
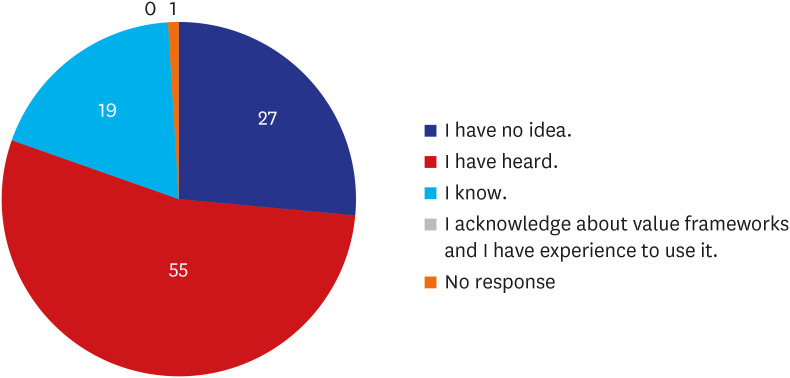
The survey shows that 90% of the respondents choose clinical efficacy as the most important value in cancer drugs selection, and the cost of drug was more weighted value in immune checkpoint inhibitors (13.7%). Approximately half (53.9%) answered that they were aware of the existing VFs. Over 90% of respondents agreed with the need for development of a VF for cancer drugs based on Korean healthcare system and further usefulness for decisions about reimbursement issues. Seventy-one percent answered that two representative VFs (American Society Clinical Oncology-VF and European Society for Medical OncologyMagnitude of Clinical Benefit Scale) should be reflected in value measurement of cancer drugs in Korea.
Conclusion
The Korean oncologists recognized the necessity for the clinical application of VF. Further discussion between the stakeholders should be followed to alleviate the financial burden through the value-based decision making of cancer drugs.
Keyword
Figure
Reference
-
1. Yu PP. Challenges in measuring cost and value in oncology: making it personal. Value Health. 2016; 19(5):520–524. PMID: 27565267.
Article2. Medical Economics. ASCO: study examines Medicare expenditures for cancer treatments. Updated 2019. Accessed May 18, 2020. https://www.medicaleconomics.com/news/asco-study-examines-medicare-expenditures-cancer-treatments.3. Renner A, Burotto M, Rojas C. Immune checkpoint inhibitor dosing: can we go lower without compromising clinical efficacy? J Glob Oncol. 2019; 5(5):1–5.
Article4. Tappenden P, Chilcott J, Ward S, Eggington S, Hind D, Hummel S. Methodological issues in the economic analysis of cancer treatments. Eur J Cancer. 2006; 42(17):2867–2875. PMID: 17023160.
Article5. Buchanan J, Wordsworth S, Schuh A. Issues surrounding the health economic evaluation of genomic technologies. Pharmacogenomics. 2013; 14(15):1833–1847. PMID: 24236483.
Article6. Cherny NI, Sullivan R, Dafni U, Kerst JM, Sobrero A, Zielinski C, et al. A standardised, generic, validated approach to stratify the magnitude of clinical benefit that can be anticipated from anti-cancer therapies: the European Society for Medical Oncology Magnitude of Clinical Benefit Scale (ESMO-MCBS). Ann Oncol. 2015; 26(8):1547–1573. PMID: 26026162.
Article7. Cherny NI, Dafni U, Bogaerts J, Latino NJ, Pentheroudakis G, Douillard JY, et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol. 2017; 28(10):2340–2366. PMID: 28945867.
Article8. Schnipper LE, Davidson NE, Wollins DS, Tyne C, Blayney DW, Blum D, et al. American Society of Clinical Oncology statement: a conceptual framework to assess the value of cancer treatment options. J Clin Oncol. 2015; 33(23):2563–2577. PMID: 26101248.
Article9. Schnipper LE, Davidson NE, Wollins DS, Blayney DW, Dicker AP, Ganz PA, et al. Updating the American society of clinical oncology value framework: revisions and reflections in response to comments received. J Clin Oncol. 2016; 34(24):2925–2934. PMID: 27247218.
Article10. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines) with NCCN Evidence Blocks. Updated 2020. Accessed May 18, 2020. https://www.nccn.org/evidenceblocks/.11. DrugAbacus. Drug Pricing Lab. Updated 2020. Accessed May 18, 2020. https://drugpricinglab.org/tools/drug-abacus/.12. Institute for Clinical and Economic Review. Updated 2020. Accessed May 18, 2020. https://icer-review.org/.13. Vokinger KN, Hwang TJ, Grischott T, Reichert S, Tibau A, Rosemann T, et al. Prices and clinical benefit of cancer drugs in the USA and Europe: a cost-benefit analysis. Lancet Oncol. 2020; 21(5):664–670. PMID: 32359489.
Article14. Medigate News. Necessity of post management after drug approval and reimbursement. Updated 2020. Accessed May 18, 2020. https://www.medigatenews.com/news/3188596286.15. Health Insurance Review and Assessment Service. Status of claims for paid drugs. Updated 2020. Accessed May 18, 2020. http://www.hira.or.kr/sViewer/index.do?ebookSn=537.16. Korean Federation of Science and Technology Societies. What is the priority for expensive anticancer drugs? Updated 2020. Accessed May 18, 2020. https://www.kofst.or.kr.17. Claims for target therapy in cancer, doubled in six years. Updated 2017. Accessed May 18, 2020. http://www.newsmp.com/news/articleView.html?idxno=178899.18. Medical News. Cancer patient status survey results. Updated 2020. Accessed May 18, 2020. http://www.mdon.co.kr.19. Gong JR, Lee D, Lim KM, Bae S. Are recently evaluated drugs more likely to receive positive reimbursement recommendations in South Korea? 11-year experience of the South Korean positive list system. Clin Ther. 2020; 42(7):1222–1233. PMID: 32487429.
Article20. Frois C, Howe A, Jarvis J, Grice K, Wong K, Zacker C, et al. Drug treatment value in a changing oncology landscape: a literature and provider perspective. J Manag Care Spec Pharm. 2019; 25(2):246–259. PMID: 30698093.
Article21. Sulmasy LS, Bledsoe TA. American college of physicians ethics manual: seventh edition. Ann Intern Med. 2019; 170(2 Suppl):S1–32.22. OBR. Value tools at ASCO 2016: building a framework for prime time. Updated 2016. Accessed May 18, 2020. https://www.obroncology.com/article/value-tools-at-asco-2016-building-a-framework-for-prime-time-2.23. Shah-Manek B, Wong W, Ravelo A, DiBonaventura M. Oncologists' perceptions of drug affordability using NCCN evidence blocks: results from a national survey. J Manag Care Spec Pharm. 2018; 24(6):565–571. PMID: 29451078.
Article24. Schnipper LE, Bastian A. New frameworks to assess value of cancer care: strengths and limitations. Oncologist. 2016; 21(6):654–658. PMID: 27245568.
Article25. Angelis A, Kanavos P. Critique of the American Society of Clinical Oncology value assessment framework for cancer treatments: Putting methodologic robustness first. J Clin Oncol. 2016; 34(24):2935–2936. PMID: 27298421.
Article26. Sobrero A, Puccini A, Bregni G, Bruzzi P. The urgent need to improve the tools to assess clinical benefit and value of cancer treatment. Eur J Cancer. 2017; 83:324–328. PMID: 28751070.
Article27. Cherny NI, de Vries EG, Dafni U, Garrett-Mayer E, McKernin SE, Piccart M, et al. Comparative assessment of clinical benefit using the ESMO-magnitude of clinical benefit scale version 1.1 and the ASCO Value Framework Net Health Benefit score. J Clin Oncol. 2019; 37(4):336–349. PMID: 30707056.
Article28. Westrich K, Buelt L, editors. Current Landscape: Value Assessment Frameworks. Washington, D.C.: National Pharmaceutical Council;2016.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Nephrotoxicity of cancer therapeutic drugs: Focusing on novel agents
- Number of Radiation Oncologists in Korea, Adequate or Surplus?
- Current landscape and future perspective of sentinel node mapping in endometrial cancer
- What is the role of surgical oncologist in the treatment of gastric cancer?
- Overview of fuzuloparib in the treatment of ovarian cancer: background and future perspective