Yeungnam Univ J Med.
2021 Jul;38(3):175-182. 10.12701/yujm.2020.00801.
Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL)
- Affiliations
-
- 1Department of Plastic and Reconstructive Surgery, Yeungnam University College of Medicine, Daegu, Korea
- KMID: 2518658
- DOI: http://doi.org/10.12701/yujm.2020.00801
Abstract
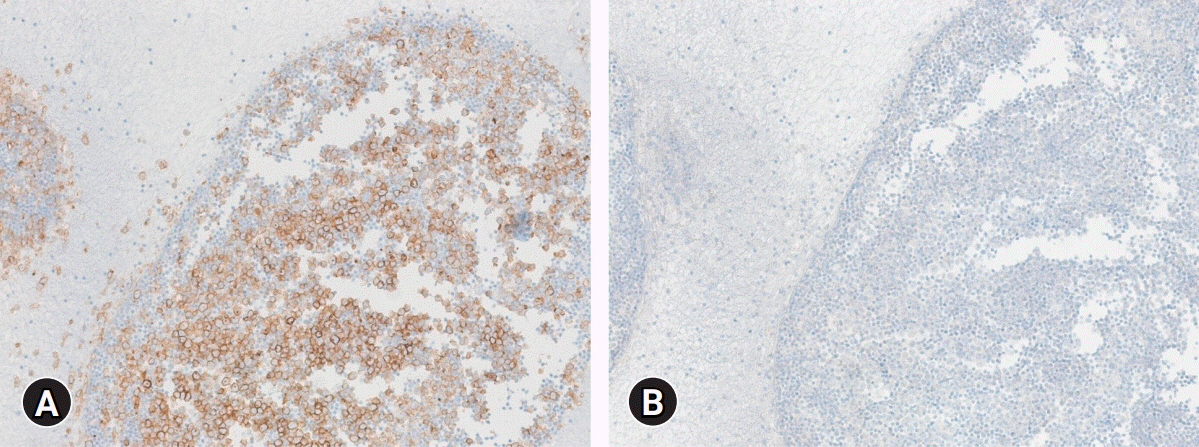
- Breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL) is a rare T-cell non-Hodgkin lymphoma characterized as CD30 positive and anaplastic lymphoma kinase (ALK) negative. In 2016, the World Health Organization declared BIA-ALCL as a new disease entity. The first case of BIA-ALCL was reported in 1997, and as of July 2019, the United States Food and Drug Administration had cited a total of 573 United States and global medical device reports of BIA-ALCL, including 33 deaths. In all clinical case reports, except for those with unknown clinical history, the patient had received at least one textured surface breast implant. Although the etiology is not yet clear, chronic inflammation has been proposed as a potential precursor to tumorigenesis. The most common presentation of BIA-ALCL is peri-implant fluid collection following aesthetic or reconstructive implantation with textured surface breast implants. It can be accompanied by breast swelling, asymmetry, pain, skin lesions, lymphadenopathy, and B-type symptoms. Most cases are detected on average 7 to 10 years after implantation. Diagnostic specimens can be obtained with fine-needle aspiration or biopsy. BIA-ALCL is CD30 positive, epithelial membrane antigen positive, and ALK negative. It can be cured with complete surgical excision at the T1–T3 stage.
Figure
Reference
-
References
1. Ministry of Food and Drug Safety; Korean Society of Plastic and Reconstructive Surgeons. The report of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in Korea [Internet]. Cheongju, KR: Ministry of Food and Drug Safety;2019. [cited 2020 May 15]. https://www.mfds.go.kr/brd/m_99/view.do?seq=43641.2. Gye SH. Unfinished fear for Allergan artificial breast: the third rare cancer in Korea occurs [Internet]. Seoul, KR: Yonhap News;2020. [cited 2020 Oct 15]. https://www.yna.co.kr/view/AKR20201004030000017?input=1195m.3. Center for Devices and Radiological Health (CDRH). Anaplastic large cell lymphoma (ALCL) in women with breast implants: preliminary FDA findings and analyses [Internet]. Silver Spring (MD): CDRH;2011. [cited 2020 May 15]. https://www.nvpc.nl/uploads/stand/NVPC110126DOC-FN-ASPS_Final_ALCL_White_Paper_Clean_Version_1-18-1177.pdf.4. Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016; 127:2375–90.
Article5. Keech JA Jr, Creech BJ. Anaplastic T-cell lymphoma in proximity to a saline-filled breast implant. Plast Reconstr Surg. 1997; 100:554–5.
Article6. U.S. Food and Drug Adiministration (FDA). Medical device reports of breast implant-associated anaplastic large cell lymphoma [Internet]. Silver Spring (MD): FDA;2020. [cited 2020 May 15]. https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma.7. de Jong D, Vasmel WL, de Boer JP, Verhave G, Barbé E, Casparie MK, et al. Anaplastic large-cell lymphoma in women with breast implants. JAMA. 2008; 300:2030–5.
Article8. Doren EL, Miranda RN, Selber JC, Garvey PB, Liu J, Medeiros LJ, et al. U.S. epidemiology of breast implant-associated anaplastic large cell lymphoma. Plast Reconstr Surg. 2017; 139:1042–50.
Article9. Loch-Wilkinson A, Beath KJ, Knight RJ, Wessels WL, Magnusson M, Papadopoulos T, et al. Breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand: high-surface-area textured implants are associated with increased risk. Plast Reconstr Surg. 2017; 140:645–54.10. Brody GS, Deapen D, Taylor CR, Pinter-Brown L, House-Lightner SR, Andersen JS, et al. Anaplastic large cell lymphoma occurring in women with breast implants: analysis of 173 cases. Plast Reconstr Surg. 2015; 135:695–705.11. Magnusson M, Beath K, Cooter R, Locke M, Prince HM, Elder E, et al. The epidemiology of breast implant-associated anaplastic large cell lymphoma in Australia and New Zealand confirms the highest risk for grade 4 surface breast implants. Plast Reconstr Surg. 2019; 143:1285–92.
Article12. Santanelli di Pompeo F, Sorotos M. EURAPS editorial: BIA-ALCL, a brief overview. J Plast Reconstr Aesthet Surg. 2018; 71:785–7.
Article13. Giot JP, Paek LS, Nizard N, El-Diwany M, Gaboury LA, Nelea M, et al. The double capsules in macro-textured breast implants. Biomaterials. 2015; 67:65–72.
Article14. Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis. 2009; 30:1073–81.
Article15. Hu H, Jacombs A, Vickery K, Merten SL, Pennington DG, Deva AK. Chronic biofilm infection in breast implants is associated with an increased T-cell lymphocytic infiltrate: implications for breast implant-associated lymphoma. Plast Reconstr Surg. 2015; 135:319–29.16. Tardío JC, Granados R. Axillary lymphadenopathy: an outstanding presentation for breast implant-associated ALK-negative anaplastic large cell lymphoma. Int J Surg Pathol. 2015; 23:424–8.17. Carty MJ, Pribaz JJ, Antin JH, Volpicelli ER, Toomey CE, Farkash EA, et al. A patient death attributable to implant-related primary anaplastic large cell lymphoma of the breast. Plast Reconstr Surg. 2011; 128:112e–118e.
Article18. Ebner PJ, Liu A, Gould DJ, Patel KM. Breast implant-associated anaplastic large cell lymphoma, a systematic review and in-depth evaluation of the current understanding. J Surg Oncol. 2019; 120:573–7.
Article19. Kricheldorff J, Fallenberg EM, Solbach C, Gerber-Schäfer C, Rancsó C, Fritschen UV. Breast implant-associated lymphoma. Dtsch Arztebl Int. 2018; 115:628–35.
Article20. Leberfinger AN, Behar BJ, Williams NC, Rakszawski KL, Potochny JD, Mackay DR, et al. Breast implant-associated anaplastic large cell lymphoma: a systematic review. JAMA Surg. 2017; 152:1161–8.
Article21. Clemens MW, Horwitz SM. NCCN consensus guidelines for the diagnosis and management of breast implant-associated anaplastic large cell lymphoma. Aesthet Surg J. 2017; 37:285–9.
Article22. Wohlgemuth FB, Brasil MB, d’Acampora AJ. Risk of breast implant-associated anaplastic large cell lymphoma in patients submitted to breast implantation: a systematic review. Breast J. 2019; 25:932–7.
Article23. Wu D, Allen CT, Fromm JR. Flow cytometry of ALK-negative anaplastic large cell lymphoma of breast implant-associated effusion and capsular tissue. Cytometry B Clin Cytom. 2015; 88:58–63.
Article24. Talagas M, Uguen A, Charles-Petillon F, Conan-Charlet V, Marion V, Hu W, et al. Breast implant-associated anaplastic large-cell lymphoma can be a diagnostic challenge for pathologists. Acta Cytol. 2014; 58:103–7.
Article25. Kim B, Predmore ZS, Mattke S, van Busum K, Gidengil CA. Breast implant-associated anaplastic large cell lymphoma: updated results from a structured expert consultation process. Plast Reconstr Surg Glob Open. 2015; 3:e296.26. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014; 32:3059–68.
Article27. Clemens MW, Medeiros LJ, Butler CE, Hunt KK, Fanale MA, Horwitz S, et al. Complete surgical excision is essential for the management of patients with breast implant-associated anaplastic large-cell lymphoma. J Clin Oncol. 2016; 34:160–8.28. Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, et al. Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients. J Clin Oncol. 2014; 32:114–20.
Article29. Estes CF, Zhang D, Reyes R, Korentager R, McGinness M, Lominska C. Locally advanced breast implant-associated anaplastic large-cell lymphoma: a case report of successful treatment with radiation and chemotherapy. Front Oncol. 2015; 5:26.
Article30. Johnson L, O’Donoghue JM, McLean N, Turton P, Khan AA, Turner SD, et al. Breast implant associated anaplastic large cell lymphoma: the UK experience: recommendations on its management and implications for informed consent. Eur J Surg Oncol. 2017; 43:1393–401.
Article31. Duvic M, Tetzlaff MT, Gangar P, Clos AL, Sui D, Talpur R. Results of a phase II trial of brentuximab vedotin for CD30+ cutaneous T-cell lymphoma and lymphomatoid papulosis. J Clin Oncol. 2015; 33:3759–65.32. Kim YH, Tavallaee M, Sundram U, Salva KA, Wood GS, Li S, et al. Phase II investigator-initiated study of brentuximab vedotin in mycosis fungoides and Sézary syndrome with variable CD30 expression level: a multi-institution collaborative project. J Clin Oncol. 2015; 33:3750–8.
Article33. Prince HM, Kim YH, Horwitz SM, Dummer R, Scarisbrick J, Quaglino P, et al. Brentuximab vedotin or physician’s choice in CD30-positive cutaneous T-cell lymphoma (ALCANZA): an international, open-label, randomised, phase 3, multicentre trial. Lancet. 2017; 390:555–66.34. Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab vedotin (SGN-35) for relapsed CD30-positive lymphomas. N Engl J Med. 2010; 363:1812–21.
Article35. Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab vedotin (SGN-35) in patients with relapsed or refractory systemic anaplastic large-cell lymphoma: results of a phase II study. J Clin Oncol. 2012; 30:2190–6.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Breast implant-associated anaplastic large cell lymphoma in an Asian patient: The first case report from Thailand
- Silicone-induced granuloma of breast implant capsule mistaken for breast implant-associated anaplastic large cell lymphoma
- Late seroma complication in a breast augmentation patient with fibrin accumulation: a case report
- Current status of breast implantassociated anaplastic large cell lymphoma in South Korea
- Undifferentiated Pleomorphic Sarcoma Mimicking Breast Implant-Associated Anaplastic Large Cell Lymphoma