J Pathol Transl Med.
2021 Jul;55(4):265-270. 10.4132/jptm.2021.06.14.
Palmar and plantar fibromatosis: a review
- Affiliations
-
- 1Department of Pathology, Immunology and Laboratory Medicine, University of Florida College of Medicine, Gainesville, FL, USA
- 2CWRU School of Medicine, University Hospitals, Bone and Soft Tissue Pathology, Cleveland, OH, USA
- KMID: 2518426
- DOI: http://doi.org/10.4132/jptm.2021.06.14
Abstract
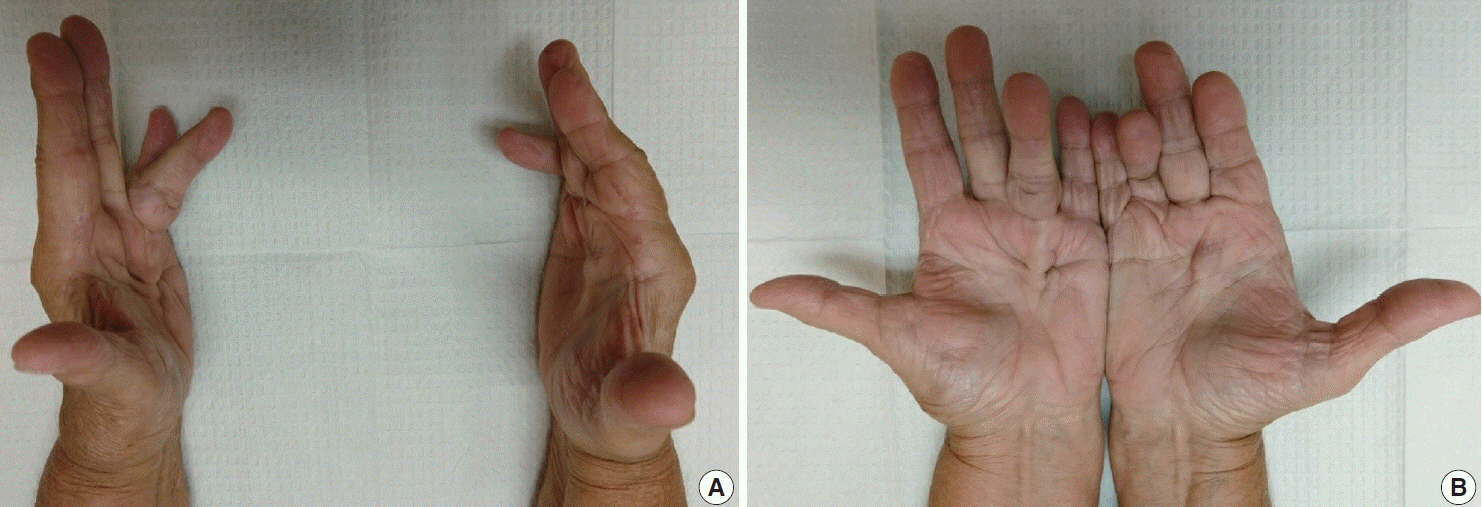
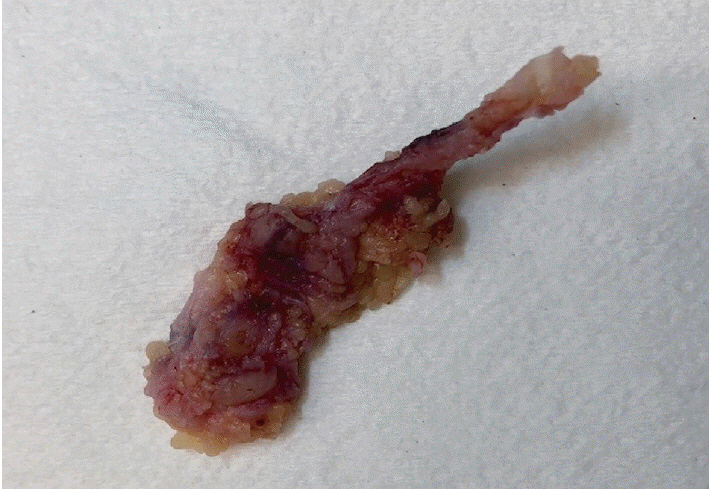
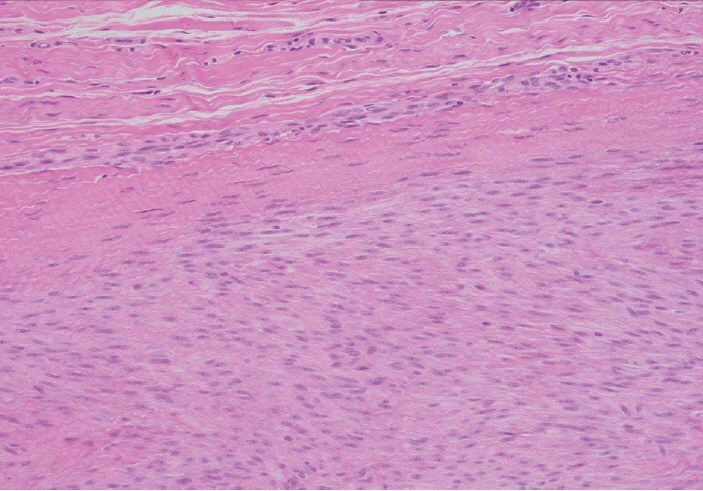
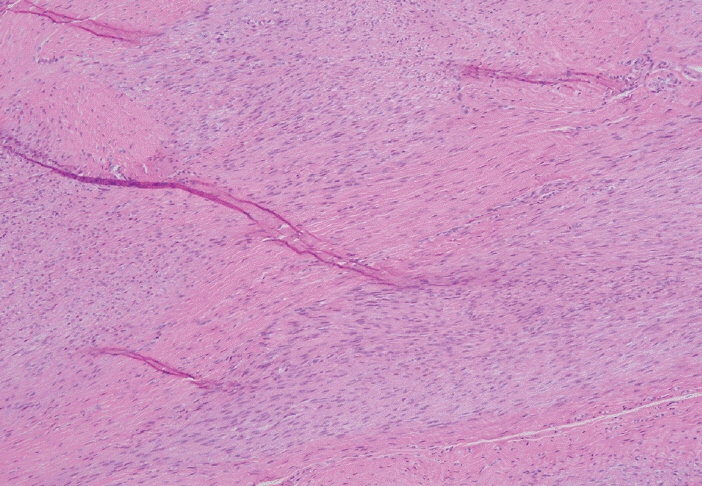
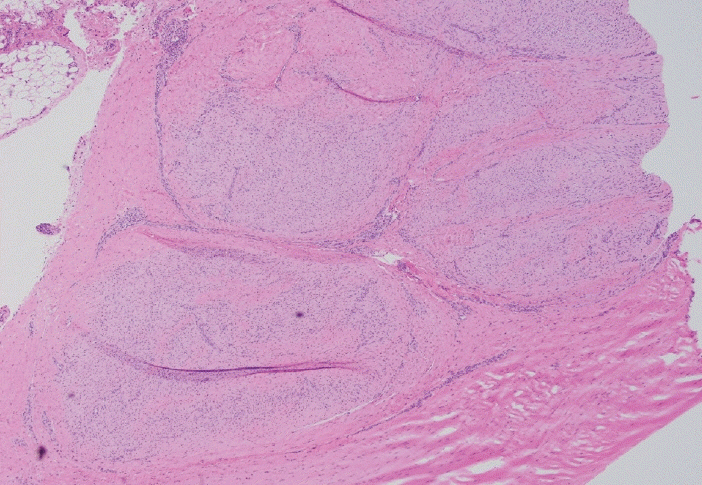
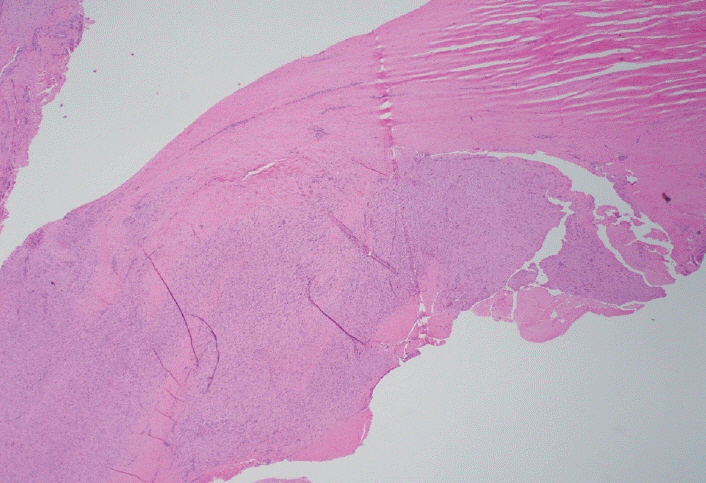
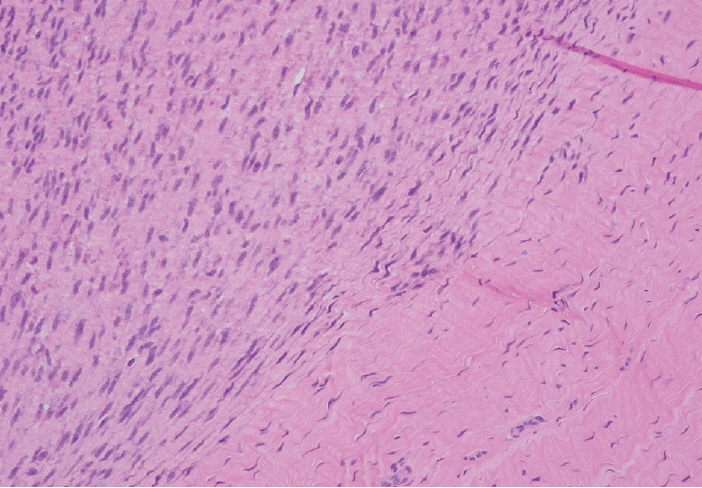
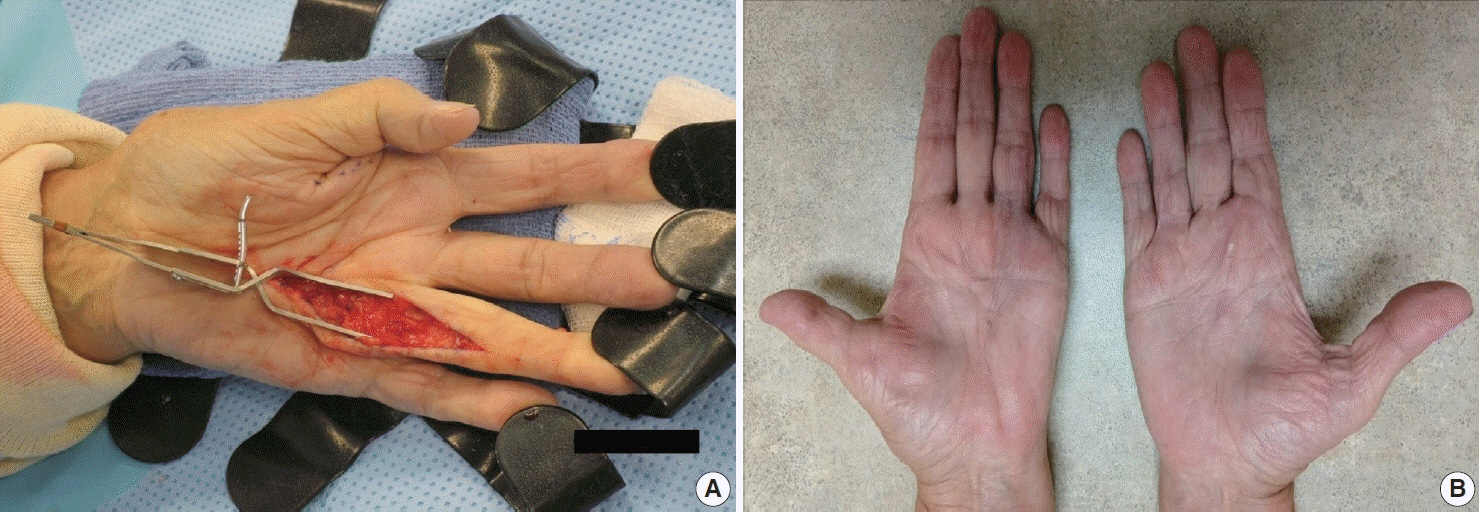
- Palmar fibromatosis (Dupuytren disease/contracture) is the most common type of fibromatosis, defined as a benign proliferation of fibroblasts and myofibroblasts. The disease process is most common in white, middle-aged and older men occurring at the distal palmar crease leading to nodules and contracture, which in many cases recur after surgical treatment. In a similar process, plantar fibromatosis (Ledderhose disease) is a proliferation of fibroblasts and myofibroblasts on the plantar aponeurosis of mostly middle-aged patients that may lead to painful nodules but usually does not lead to contracture. Both processes are histologically similar, composed of a bland cellular proliferation of spindle cells with a bluish appearance and with a variable amount of background collagen, depending on the age of the lesion. The etiology of both lesions is still uncertain, while treatment ranges from observation to surgery, with some pharmacologic agents being investigated with mixed success. In this paper we provide an overview of both processes with regards to clinical and radiologic findings, pathophysiology, diagnosis, treatment, and prognosis.
Keyword
Figure
Reference
-
References
1. Dibenedetti DB, Nguyen D, Zografos L, Ziemiecki R, Zhou X. Prevalence, incidence, and treatments of Dupuytren’s disease in the United States: results from a population-based study. Hand (N Y). 2011; 6:149–58.
Article2. Cordova A, Tripoli M, Corradino B, Napoli P, Moschella F. Dupuytren’s contracture: an update of biomolecular aspects and therapeutic perspectives. J Hand Surg Br. 2005; 30:557–62.
Article3. Follonier Castella L, Gabbiani G, McCulloch CA, Hinz B. Regulation of myofibroblast activities: calcium pulls some strings behind the scene. Exp Cell Res. 2010; 316:2390–401.
Article4. Krause C, Kloen P. Concurrent inhibition of TGF-beta and mitogen driven signaling cascades in Dupuytren’s disease: non-surgical treatment strategies from a signaling point of view. Med Hypotheses. 2012; 78:385–8.5. Ratkaj I, Bujak M, Jurisic D, et al. Microarray analysis of Dupuytren’s disease cells: the profibrogenic role of the TGF-beta inducible p38 MAPK pathway. Cell Physiol Biochem. 2012; 30:927–42.6. Dolmans GH, Werker PM, Hennies HC, et al. Wnt signaling and Dupuytren’s disease. N Engl J Med. 2011; 365:307–17.
Article7. Sayadi LR, Alhunayan D, Sarantopoulos N, et al. The molecular pathogenesis of Dupuytren disease: review of the literature and suggested new approaches to treatment. Ann Plast Surg. 2019; 83:594–600.8. Carroll P, Henshaw RM, Garwood C, Raspovic K, Kumar D. Plantar fibromatosis: pathophysiology, surgical and nonsurgical therapies: an evidence-based review. Foot Ankle Spec. 2018; 11:168–76.
Article9. Godette GA, O'Sullivan M, Menelaus MB. Plantar fibromatosis of the heel in children: a report of 14 cases. J Pediatr Orthop. 1997; 17:16–7.
Article10. Young JR, Sternbach S, Willinger M, Hutchinson ID, Rosenbaum AJ. The etiology, evaluation, and management of plantar fibromatosis. Orthop Res Rev. 2019; 11:1–7.
Article11. Kim SK, Ioannidis JPA, Ahmed MA, et al. Two genetic variants associated with plantar fascial disorders. Int J Sports Med. 2018; 39:314–21.
Article12. Strzelczyk A, Vogt H, Hamer HM, Kramer G. Continuous phenobarbital treatment leads to recurrent plantar fibromatosis. Epilepsia. 2008; 49:1965–8.
Article13. Teh J. Ultrasound of soft tissue masses of the hand. J Ultrason. 2012; 12:381–401.
Article14. Yacoe ME, Bergman AG, Ladd AL, Hellman BH. Dupuytren's contracture: MR imaging findings and correlation between MR signal intensity and cellularity of lesions. AJR Am J Roentgenol. 1993; 160:813–7.
Article15. Espert M, Anderson MR, Baumhauer JF. Current concepts review: plantar fibromatosis. Foot Ankle Int. 2018; 39:751–7.
Article16. McNally EG, Shetty S. Plantar fascia: imaging diagnosis and guided treatment. Semin Musculoskelet Radiol. 2010; 14:334–43.
Article17. Griffith JF, Wong TY, Wong SM, Wong MW, Metreweli C. Sonography of plantar fibromatosis. AJR Am J Roentgenol. 2002; 179:1167–72.
Article18. Cohen BE, Murthy NS, McKenzie GA. Ultrasonography of plantar fibromatosis: updated case series, review of the literature, and a novel descriptive appearance termed the “Comb sign”. J Ultrasound Med. 2018; 37:2725–31.
Article19. Narvaez JA, Narvaez J, Ortega R, Aguilera C, Sanchez A, Andia E. Painful heel: MR imaging findings. Radiographics. 2000; 20:333–52.
Article20. Evans HL. Multinucleated giant cells in plantar fibromatosis. Am J Surg Pathol. 2002; 26:244–8.
Article21. Carlson JW, Fletcher CD. Immunohistochemistry for beta-catenin in the differential diagnosis of spindle cell lesions: analysis of a series and review of the literature. Histopathology. 2007; 51:509–14.22. Fausto de Souza D, Micaelo L, Cuzzi T, Ramos ESM. Ledderhose disease: an unusual presentation. J Clin Aesthet Dermatol. 2010; 3:45–7.23. Wang L, Zhu H. Clonal analysis of palmar fibromatosis: a study whether palmar fibromatosis is a real tumor. J Transl Med. 2006; 4:21.
Article24. Kaur S, Forsman M, Ryhanen J, Knuutila S, Larramendy ML. No gene copy number changes in Dupuytren’s contracture by array comparative genomic hybridization. Cancer Genet Cytogenet. 2008; 183:6–8.
Article25. De Wever I, Dal Cin P, Fletcher CD, et al. Cytogenetic, clinical, and morphologic correlations in 78 cases of fibromatosis: a report from the CHAMP Study Group. CHromosomes And Morphology. Mod Pathol. 2000; 13:1080–5.26. Shih B, Tassabehji M, Watson JS, Bayat A. DNA copy number variations at chromosome 7p14.1 and chromosome 14q11.2 are associated with Dupuytren’s disease: potential role for MMP and Wnt signaling pathway. Plast Reconstr Surg. 2012; 129:921–32.27. Michou L, Lermusiaux JL, Teyssedou JP, Bardin T, Beaudreuil J, Petit- Teixeira E. Genetics of Dupuytren’s disease. Joint Bone Spine. 2012; 79:7–12.
Article28. Breiner JA, Nelson M, Bredthauer BD, Neff JR, Bridge JA. Trisomy 8 and trisomy 14 in plantar fibromatosis. Cancer Genet Cytogenet. 1999; 108:176–7.29. Sawyer JR, Sammartino G, Gokden N, Nicholas RW. A clonal reciprocal t(2;7)(p13;p13) in plantar fibromatosis. Cancer Genet Cytogenet. 2005; 158:67–9.
Article30. Denkler KA, Vaughn CJ, Dolan EL, Hansen SL. Evidence-based medicine: options for Dupuytren’s contracture: incise, excise, and dissolve. Plast Reconstr Surg. 2017; 139:240e–55e.31. Sanjuan-Cervero R. Current role of the collagenase Clostridium histolyticum in Dupuytren's disease treatment. Ir J Med Sci. 2020; 189:529–34.
Article32. Townley WA, Baker R, Sheppard N, Grobbelaar AO. Dupuytren’s contracture unfolded. BMJ. 2006; 332:397–400.
Article33. van der Veer WM, Hamburg SM, de Gast A, Niessen FB. Recurrence of plantar fibromatosis after plantar fasciectomy: single-center long-term results. Plast Reconstr Surg. 2008; 122:486–91.
Article34. Kadir HK, Chandrasekar CR. Partial fasciectomy is a useful treatment option for symptomatic plantar fibromatosis. Foot (Edinb). 2017; 31:31–4.
Article