J Neurocrit Care.
2021 Jun;14(1):46-51. 10.18700/jnc.200025.
Refractory brainstem encephalitis mimicking progressive cerebral infarction: infliximab and methotrexate as a salvage immunotherapy
- Affiliations
-
- 1Department of Neurology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Busan, Republic of Korea
- 2Department of Radiology, Research Institute for Convergence of Biomedical Science and Technology, Pusan National University Yangsan Hospital, Pusan National University School of Medicine, Busan, Republic of Korea
- KMID: 2517132
- DOI: http://doi.org/10.18700/jnc.200025
Abstract
- Background
Brainstem encephalitis is a rare, severe, and potentially life-threatening inflammation of the central nervous system, exhibiting various treatment responses and outcomes owing to multiple etiologies.
Case Report
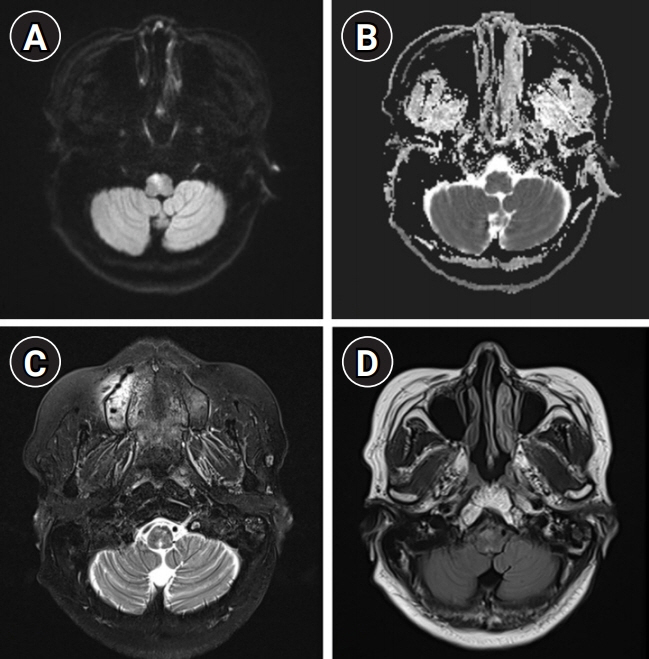
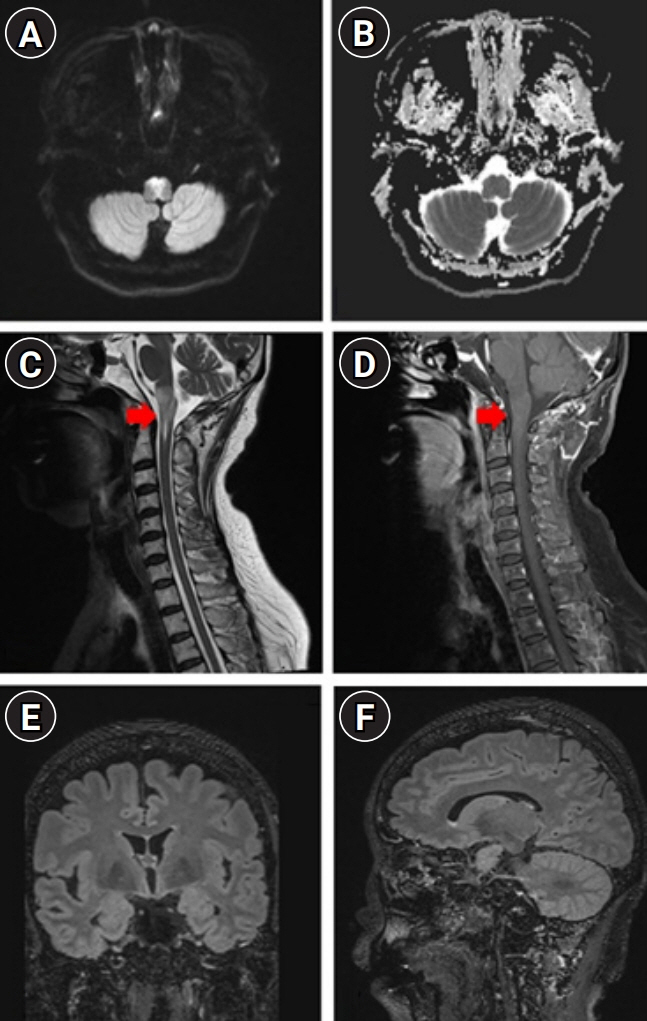
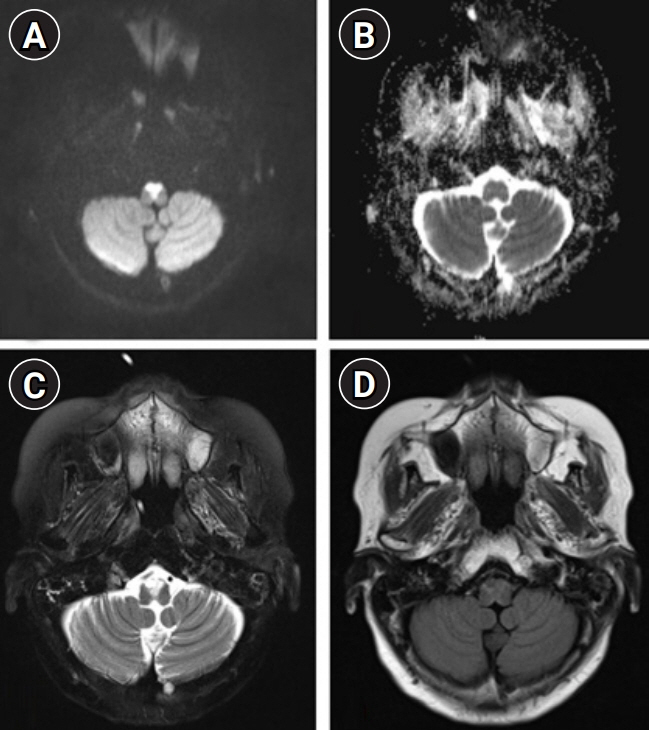
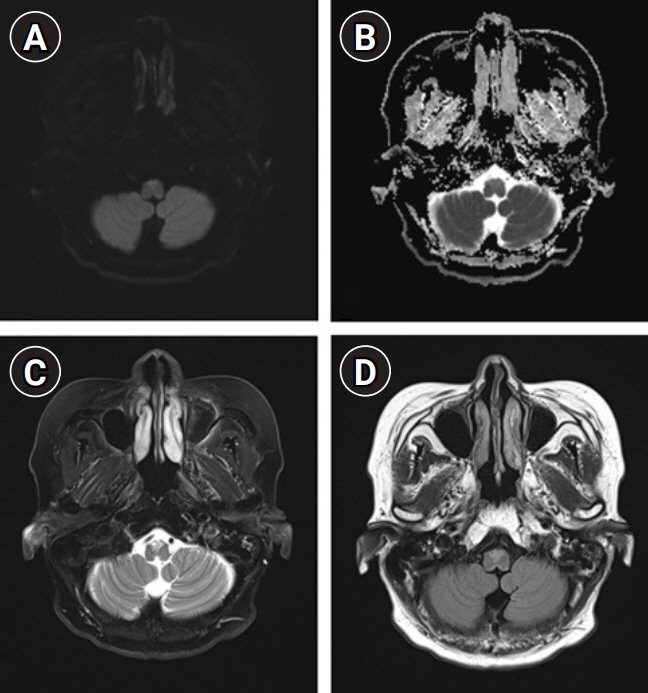
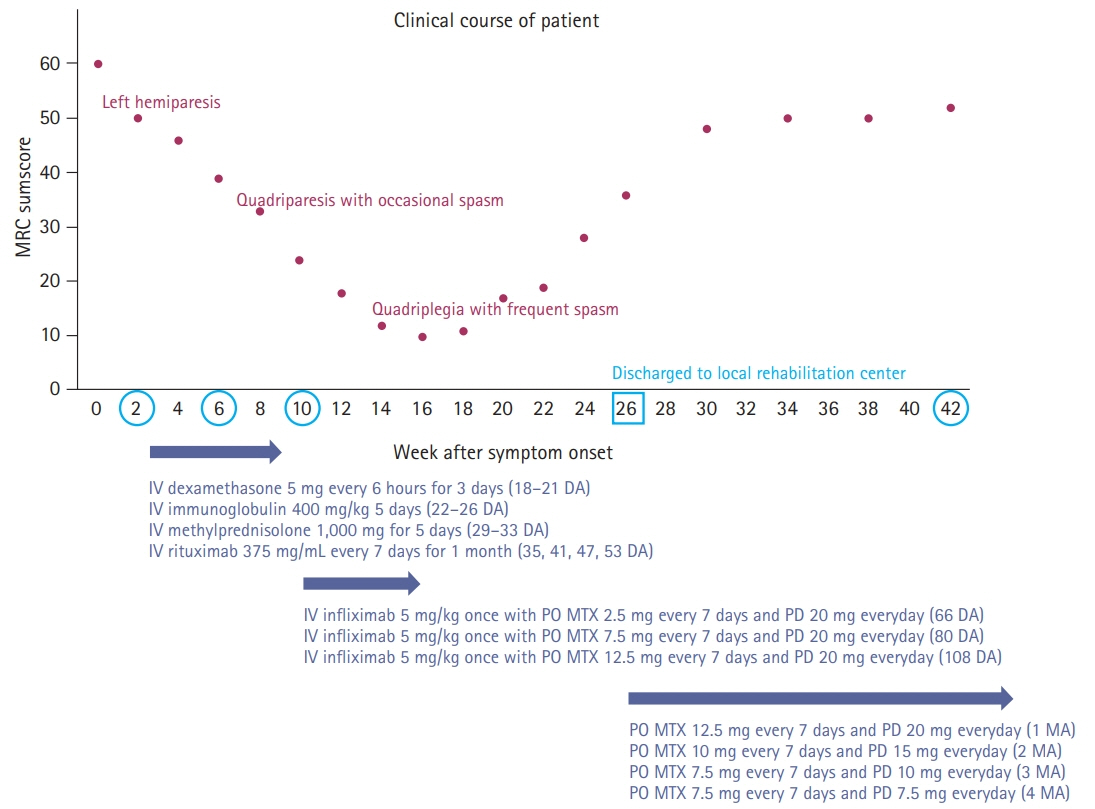
We describe the favorable outcome of salvage immunotherapy using a combination of infliximab and methotrexate in a 62-year-old woman with refractory brainstem encephalitis. The patient was initially presumed to be at a subacute stage of medullary infarction but showed progressively worsening conditions involving cervical myelopathy, despite having completed the schedule of subsequent immunotherapy with intravenous methylprednisolone, immunoglobulin, and rituximab. After completion of four sessions of weekly rituximab injection, she was treated with 5 mg/kg of infliximab, scheduled at 0, 2, and 6 weeks, along with methotrexate (weekly 12.5 mg). After completion of infliximab injection and maintenance with methotrexate treatment, she showed an improving course of quadriplegia.
Conclusion
This case report provides evidence for the potential efficacy of infliximab with methotrexate in cases of refractory brainstem encephalitis.
Figure
Reference
-
1. Tan IL, Mowry EM, Steele SU, Pardo CA, McArthur JC, Nath A, et al. Brainstem encephalitis: etiologies, treatment, and predictors of outcome. J Neurol. 2013; 260:2312–9.
Article2. Bin Abdulqader SA, Alkhalidi HM, Ajlan AM. Brainstem encephalitis: a diagnostic dilemma. Neurosciences (Riyadh). 2018; 23:152–7.3. Law LY, Riminton DS, Nguyen M, Barnett MH, Reddel SW, Hardy TA. The spectrum of immune-mediated and inflammatory lesions of the brainstem: clues to diagnosis. Neurology. 2019; 93:390–405.4. Overell JR, Hsieh ST, Odaka M, Yuki N, Willison HJ. Treatment for Fisher syndrome, Bickerstaff's brainstem encephalitis and related disorders. Cochrane Database Syst Rev. 2007; (1):CD004761.
Article5. Hermetter C, Fazekas F, Hochmeister S. Systematic review: syndromes, early diagnosis, and treatment in autoimmune encephalitis. Front Neurol. 2018; 9:706.
Article6. Shin JW, Koo YS, Kim YS, Kim DW, Kim KK, Lee SY, et al. Clinical characterization of unknown/cryptogenic status epilepticus suspected as encephalitis: a multicenter cohort study. J Neuroimmunol. 2018; 315:1–8.
Article7. Saiz A, Bruna J, Stourac P, Vigliani MC, Giometto B, Grisold W, et al. Anti-Hu-associated brainstem encephalitis. J Neurol Neurosurg Psychiatry. 2009; 80:404–7.
Article8. Kamm C, Zettl UK. Autoimmune disorders affecting both the central and peripheral nervous system. Autoimmun Rev. 2012; 11:196–202.
Article9. Cohen-Gadol AA, Zikel OM, Miller GM, Aksamit AJ, Scheithauer BW, Krauss WE. Spinal cord biopsy: a review of 38 cases. Neurosurgery. 2003; 52:806–15.
Article10. Shirabe T, Tatsuta E, Kuroiwa Y, Tanaka K. An autopsy case of brain stem encephalitis with spinal cord involvement. Folia Psychiatr Neurol Jpn. 1972; 26:133–43.
Article11. Segal BM. Neurosarcoidosis: diagnostic approaches and therapeutic strategies. Curr Opin Neurol. 2013; 26:307–13.12. Gelfand JM, Bradshaw MJ, Stern BJ, Clifford DB, Wang Y, Cho TA, et al. Infliximab for the treatment of CNS sarcoidosis: a multi-institutional series. Neurology. 2017; 89:2092–100.13. Dalakas MC. B cells as therapeutic targets in autoimmune neurological disorders. Nat Clin Pract Neurol. 2008; 4:557–67.
Article14. Cronstein BN. The mechanism of action of methotrexate. Rheum Dis Clin North Am. 1997; 23:739–55.
Article15. Bhojwani D, Sabin ND, Pei D, Yang JJ, Khan RB, Panetta JC, et al. Methotrexate-induced neurotoxicity and leukoencephalopathy in childhood acute lymphoblastic leukemia. J Clin Oncol. 2014; 32:949–59.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Brainstem Encephalitis Mimicking Miller Fisher Syndrome
- Prolonged Comatose State Followed by Rapid Recovery in a Patient with Bickerstaff's Brainstem Encephalitis
- A Case of Brainstem Encephalitis Presented with Diplopia and Headache
- Infliximab: Effective Therapy for Pustular Psoriasis
- A Case of Recurrent Generalized Pustular Psoriasis Treated with a Combination of Infliximab with Methotrexate and Retinoid