MondoA Is Required for Normal Myogenesis and Regulation of the Skeletal Muscle Glycogen Content in Mice
- Affiliations
-
- 1Department of Endocrinology, Xinhua Hospital, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
- 2Department of Hematology, Renmin Hospital, Wuhan University, Wuhan, China.
- 3Department of Pediatrics, 1st Affiliated Hospital, Zhengzhou University, Zhengzhou, China.
- 4Department of Biochemistry and Molecular Cell Biology, Shanghai Key Laboratory for Tumor Microenvironment and Inflammation, Key Laboratory of Cell Differentiation and Apoptosis of Chinese Ministry of Education, Shanghai Jiao Tong University School of Medicine, Shanghai, China.
- KMID: 2516345
- DOI: http://doi.org/10.4093/dmj.2019.0212
Abstract
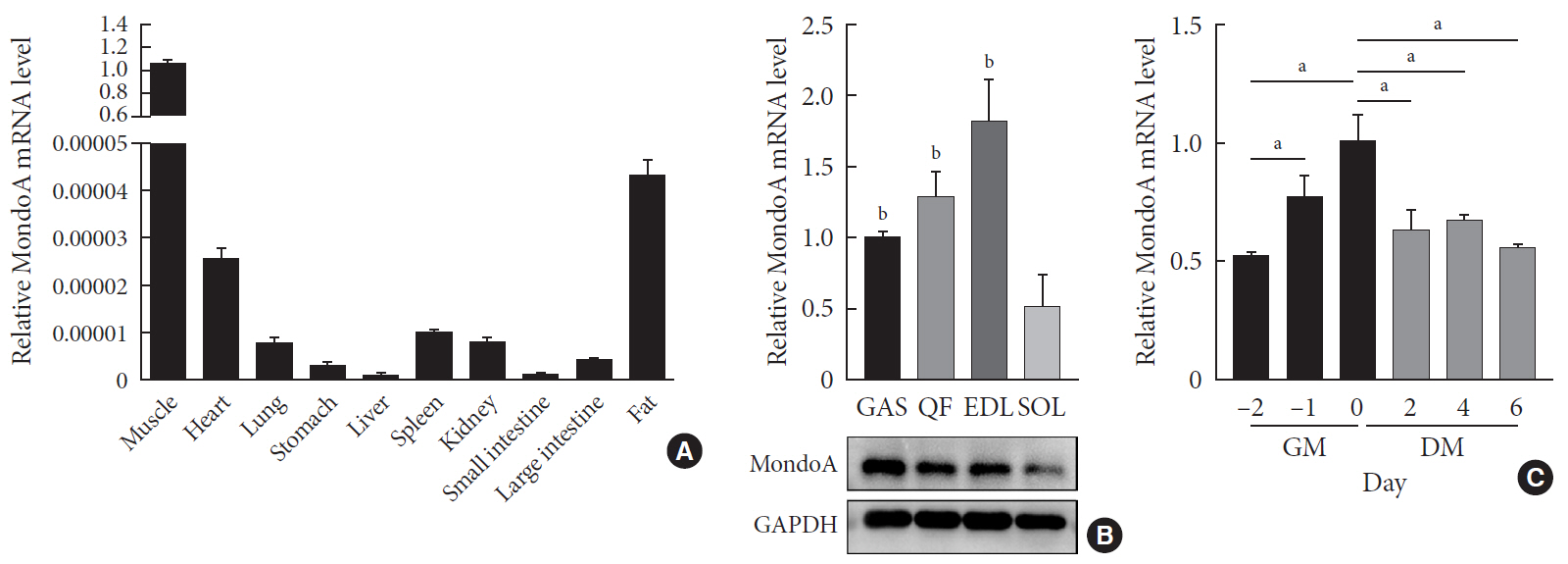
Background Skeletal muscle is the largest tissue in the human body, and it plays a major role in exerting force and maintaining metabolism homeostasis. The role of muscle transcription factors in the regulation of metabolism is not fully understood. MondoA is a glucose-sensing transcription factor that is highly expressed in skeletal muscle. Previous studies suggest that MondoA can influence systemic metabolism homeostasis. However, the function of MondoA in the skeletal muscle remains unclear.
Methods We generated muscle-specific MondoA knockout (MAKO) mice and analyzed the skeletal muscle morphology and glycogen content. Along with skeletal muscle from MAKO mice, C2C12 myocytes transfected with small interfering RNA against MondoA were also used to investigate the role and potential mechanism of MondoA in the development and glycogen metabolism of skeletal muscle.
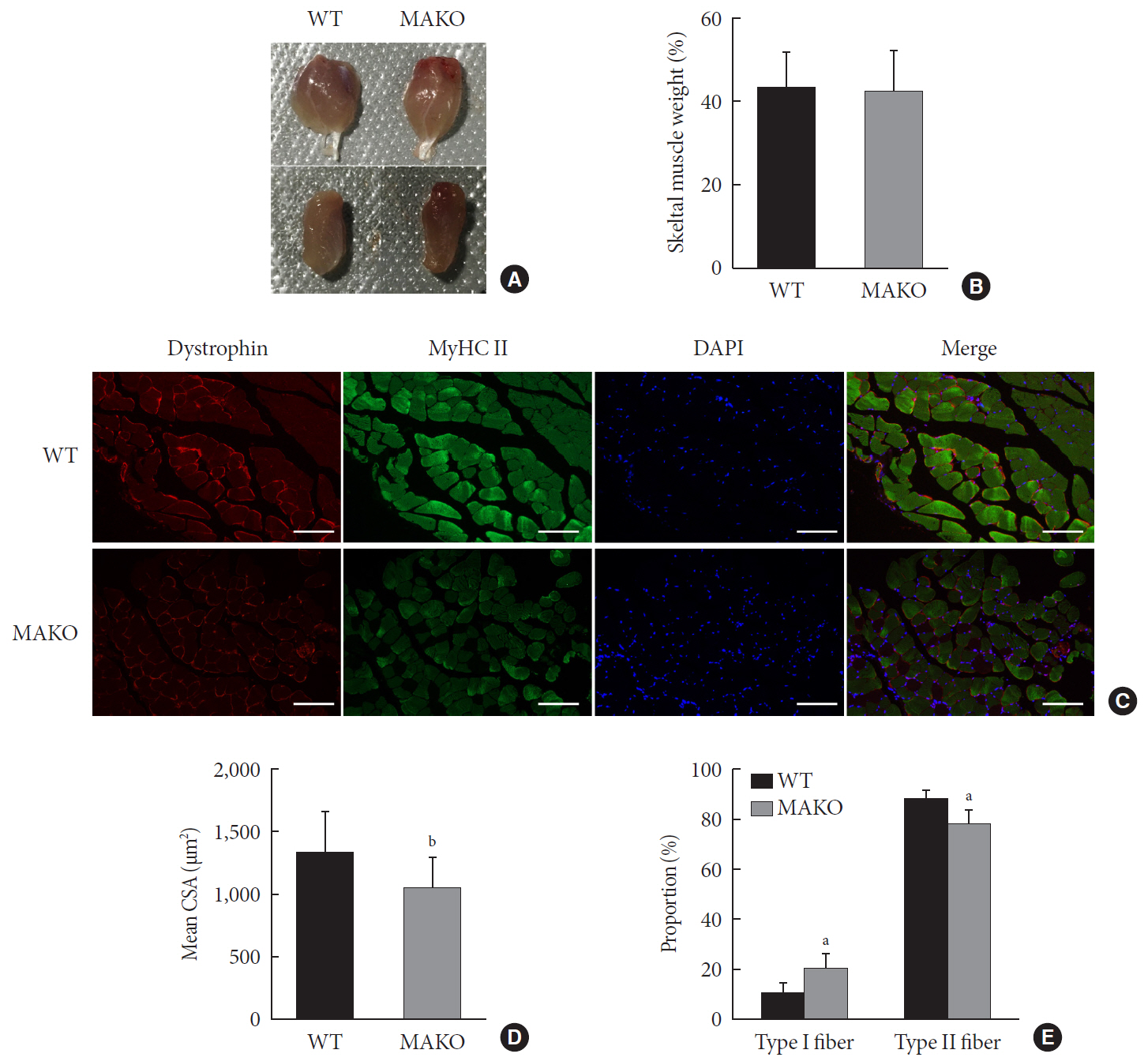
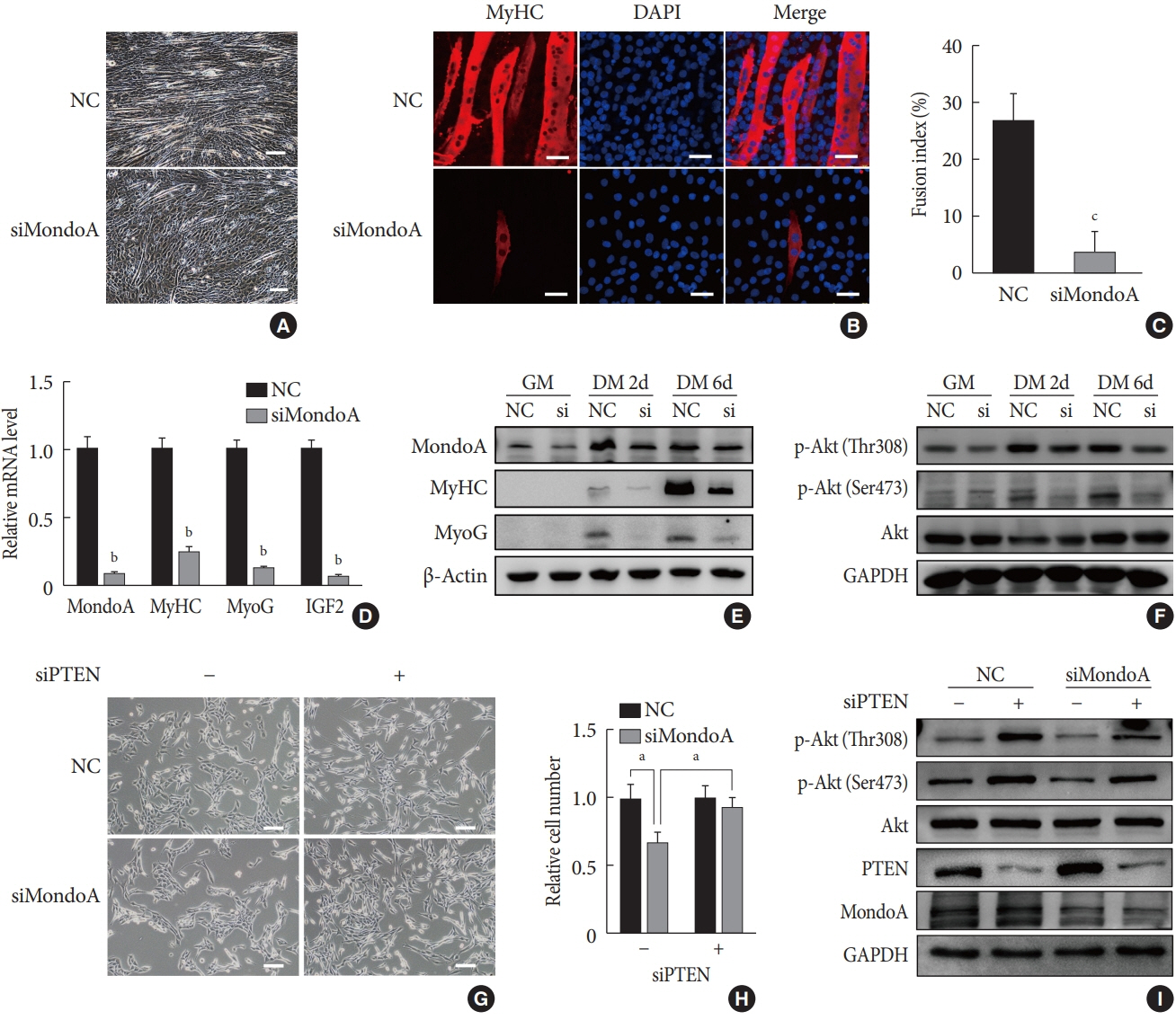
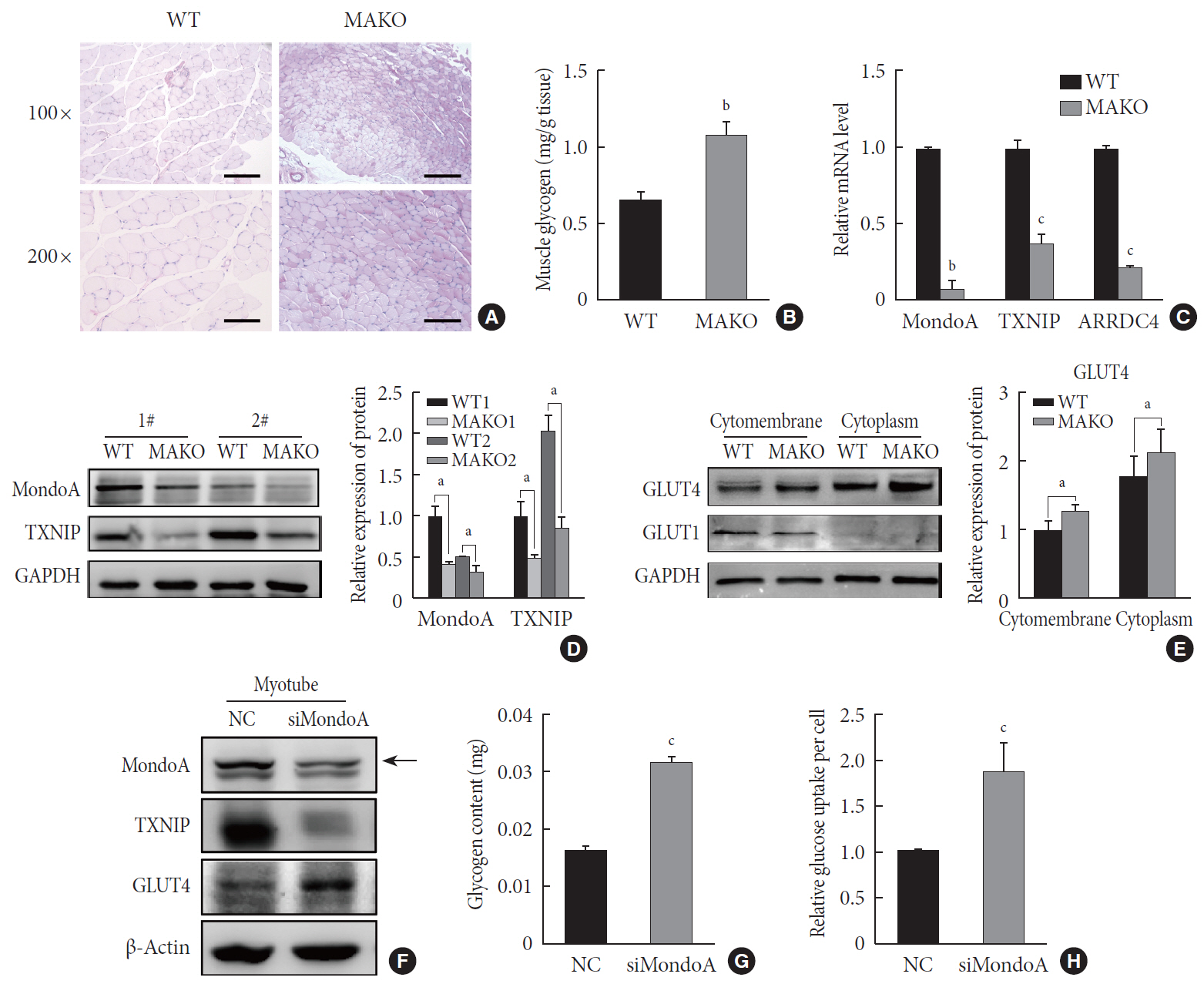
Results MAKO caused muscle fiber atrophy, reduced the proportion of type II fibers compared to type I fibers, and increased the muscle glycogen level. MondoA knockdown inhibited myoblast proliferation, migration, and differentiation by inhibiting the phosphatase and tensin homolog (PTEN)/phosphoinositide 3-kinase (PI3K)/Akt pathway. Further mechanistic experiments revealed that the increased muscle glycogen in MAKO mice was caused by thioredoxin-interacting protein (TXNIP) downregulation, which led to upregulation of glucose transporter 4 (GLUT4), potentially increasing glucose uptake.
Conclusion MondoA appears to mediate mouse myofiber development, and MondoA decreases the muscle glycogen level. The findings indicate the potential function of MondoA in skeletal muscle, linking the glucose-related transcription factor to myogenesis and skeletal myofiber glycogen metabolism.
Figure
Reference
-
1. Bassel-Duby R, Olson EN. Signaling pathways in skeletal muscle remodeling. Annu Rev Biochem. 2006; 75:19–37.
Article2. Schiaffino S. Fibre types in skeletal muscle: a personal account. Acta Physiol (Oxf). 2010; 199:451–463.
Article3. Billin AN, Eilers AL, Coulter KL, Logan JS, Ayer DE. MondoA, a novel basic helix-loop-helix-leucine zipper transcriptional activator that constitutes a positive branch of a max-like network. Mol Cell Biol. 2000; 20:8845–8854.
Article4. Li MV, Chang B, Imamura M, Poungvarin N, Chan L. Glucose-dependent transcriptional regulation by an evolutionarily conserved glucose-sensing module. Diabetes. 2006; 55:1179–1189.
Article5. Yamashita H, Takenoshita M, Sakurai M, Bruick RK, Henzel WJ, Shillinglaw W, Arnot D, Uyeda K. A glucose-responsive transcription factor that regulates carbohydrate metabolism in the liver. Proc Natl Acad Sci U S A. 2001; 98:9116–9121.
Article6. Towle HC. Glucose as a regulator of eukaryotic gene transcription. Trends Endocrinol Metab. 2005; 16:489–494.
Article7. Iizuka K, Bruick RK, Liang G, Horton JD, Uyeda K. Deficiency of carbohydrate response element-binding protein (ChREBP) reduces lipogenesis as well as glycolysis. Proc Natl Acad Sci U S A. 2004; 101:7281–7286.8. Stoltzman CA, Peterson CW, Breen KT, Muoio DM, Billin AN, Ayer DE. Glucose sensing by MondoA: Mlx complexes: a role for hexokinases and direct regulation of thioredoxin-interacting protein expression. Proc Natl Acad Sci U S A. 2008; 105:6912–6917.9. Stoltzman CA, Kaadige MR, Peterson CW, Ayer DE. MondoA senses non-glucose sugars: regulation of thioredoxin-interacting protein (TXNIP) and the hexose transport curb. J Biol Chem. 2011; 286:38027–38034.10. Ahn B, Soundarapandian MM, Sessions H, Peddibhotla S, Roth GP, Li JL, Sugarman E, Koo A, Malany S, Wang M, Yea K, Brooks J, Leone TC, Han X, Vega RB, Kelly DP. MondoA coordinately regulates skeletal myocyte lipid homeostasis and insulin signaling. J Clin Invest. 2016; 126:3567–3579.
Article11. Ran H, Zhu Y, Deng R, Zhang Q, Liu X, Feng M, Zhong J, Lin S, Tong X, Su Q. Stearoyl-CoA desaturase-1 promotes colorectal cancer metastasis in response to glucose by suppressing PTEN. J Exp Clin Cancer Res. 2018; 37:54.
Article12. Huang SL, Yu RT, Gong J, Feng Y, Dai YL, Hu F, Hu YH, Tao YD, Leng Y. Arctigenin, a natural compound, activates AMP-activated protein kinase via inhibition of mitochondria complex I and ameliorates metabolic disorders in ob/ob mice. Diabetologia. 2012; 55:1469–1481.
Article13. Bentzinger CF, Wang YX, Rudnicki MA. Building muscle: molecular regulation of myogenesis. Cold Spring Harb Perspect Biol. 2012; 4:a008342.
Article14. Sabourin LA, Rudnicki MA. The molecular regulation of myogenesis. Clin Genet. 2000; 57:16–25.
Article15. Chen X, Wan J, Yu B, Diao Y, Zhang W. PIP5K1α promotes myogenic differentiation via AKT activation and calcium release. Stem Cell Res Ther. 2018; 9:33.
Article16. Xu Q, Wu Z. The insulin-like growth factor-phosphatidylinositol 3-kinase-Akt signaling pathway regulates myogenin expression in normal myogenic cells but not in rhabdomyosarcoma-derived RD cells. J Biol Chem. 2000; 275:36750–36757.
Article17. Pearce LR, Komander D, Alessi DR. The nuts and bolts of AGC protein kinases. Nat Rev Mol Cell Biol. 2010; 11:9–22.
Article18. Shan T, Liu J, Xu Z, Wang Y. Roles of phosphatase and tensin homolog in skeletal muscle. J Cell Physiol. 2019; 234:3192–3196.
Article19. Parikh H, Carlsson E, Chutkow WA, Johansson LE, Storgaard H, Poulsen P, Saxena R, Ladd C, Schulze PC, Mazzini MJ, Jensen CB, Krook A, Bjornholm M, Tornqvist H, Zierath JR, Ridderstrale M, Altshuler D, Lee RT, Vaag A, Groop LC, Mootha VK. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 2007; 4:e158.
Article20. Kaadige MR, Looper RE, Kamalanaadhan S, Ayer DE. Glutamine-dependent anapleurosis dictates glucose uptake and cell growth by regulating MondoA transcriptional activity. Proc Natl Acad Sci U S A. 2009; 106:14878–14883.
Article21. Muoio DM. TXNIP links redox circuitry to glucose control. Cell Metab. 2007; 5:412–414.
Article22. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013; 93:993–1017.
Article23. Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011; 91:1447–1531.
Article24. Imamura M, Chang BH, Kohjima M, Li M, Hwang B, Taegtmeyer H, Harris RA, Chan L. MondoA deficiency enhances sprint performance in mice. Biochem J. 2014; 464:35–48.
Article25. Luo W, Nie Q, Zhang X. MicroRNAs involved in skeletal muscle differentiation. J Genet Genomics. 2013; 40:107–116.
Article26. Katase N, Terada K, Suzuki T, Nishimatsu S, Nohno T. miR-487b, miR-3963 and miR-6412 delay myogenic differentiation in mouse myoblast-derived C2C12 cells. BMC Cell Biol. 2015; 16:13.
Article27. De Falco M, De Luca A. Involvement of cdks and cyclins in muscle differentiation. Eur J Histochem. 2006; 50:19–23.28. Wu M, Yang G, Chen Y, Zhou X, Chen H, Li M, Yu K, Zhang X, Xie S, Zhang Y, Chu G, Mo D. CEP2 attenuates myoblast differentiation but does not affect proliferation. Int J Biol Sci. 2015; 11:99–108.
Article29. Tanaka K, Sato K, Yoshida T, Fukuda T, Hanamura K, Kojima N, Shirao T, Yanagawa T, Watanabe H. Evidence for cell density affecting C2C12 myogenesis: possible regulation of myogenesis by cell-cell communication. Muscle Nerve. 2011; 44:968–977.
Article30. Hwang SY, Kang YJ, Sung B, Jang JY, Hwang NL, Oh HJ, Ahn YR, Kim HJ, Shin JH, Yoo MA, Kim CM, Chung HY, Kim ND. Folic acid is necessary for proliferation and differentiation of C2C12 myoblasts. J Cell Physiol. 2018; 233:736–747.
Article31. Zhu M, Liu J, Xiao J, Yang L, Cai M, Shen H, Chen X, Ma Y, Hu S, Wang Z, Hong A, Li Y, Sun Y, Wang X. Lnc-mg is a long non-coding RNA that promotes myogenesis. Nat Commun. 2017; 8:14718.
Article32. Yang W, Zhang Y, Li Y, Wu Z, Zhu D. Myostatin induces cyclin D1 degradation to cause cell cycle arrest through a phosphatidylinositol 3-kinase/AKT/GSK-3 beta pathway and is antagonized by insulin-like growth factor 1. J Biol Chem. 2007; 282:3799–3808.33. Go GY, Lee SJ, Jo A, Lee JR, Kang JS, Yang M, Bae GU. Bisphenol A and estradiol impede myoblast differentiation through down-regulating Akt signaling pathway. Toxicol Lett. 2018; 292:12–19.
Article34. Yue F, Bi P, Wang C, Shan T, Nie Y, Ratliff TL, Gavin TP, Kuang S. Pten is necessary for the quiescence and maintenance of adult muscle stem cells. Nat Commun. 2017; 8:14328.
Article35. Yue F, Bi P, Wang C, Li J, Liu X, Kuang S. Conditional loss of Pten in myogenic progenitors leads to postnatal skeletal muscle hypertrophy but age-dependent exhaustion of satellite cells. Cell Rep. 2016; 17:2340–2353.
Article36. Yan S, Liu H, Liu Z, Peng F, Jiang F, Li L, Fu R. CCN1 stimulated the osteoblasts via PTEN/AKT/GSK3β/cyclinD1 signal pathway in myeloma bone disease. Cancer Med. 2020; 9:737–744.37. Cahill GF Jr. Fuel metabolism in starvation. Annu Rev Nutr. 2006; 26:1–22.
Article38. Greenberg CC, Jurczak MJ, Danos AM, Brady MJ. Glycogen branches out: new perspectives on the role of glycogen metabolism in the integration of metabolic pathways. Am J Physiol Endocrinol Metab. 2006; 291:E1–E8.
Article39. Sans CL, Satterwhite DJ, Stoltzman CA, Breen KT, Ayer DE. MondoA-Mlx heterodimers are candidate sensors of cellular energy status: mitochondrial localization and direct regulation of glycolysis. Mol Cell Biol. 2006; 26:4863–4871.
Article40. Ahn B, Wan S, Jaiswal N, Vega RB, Ayer DE, Titchenell PM, Han X, Won KJ, Kelly DP. MondoA drives muscle lipid accumulation and insulin resistance. JCI Insight. 2019; 5:e129119.
Article41. Lombardi AM, Moller D, Loizeau M, Girard J, Leturque A. Phenotype of transgenic mice overexpressing GLUT4 and hexokinase II in muscle. FASEB J. 1997; 11:1137–1144.
Article42. Mandala A, Das N, Bhattacharjee S, Mukherjee B, Mukhopadhyay S, Roy SS. Thioredoxin interacting protein mediates lipid-induced impairment of glucose uptake in skeletal muscle. Biochem Biophys Res Commun. 2016; 479:933–939.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- MondoA Is Required for Normal Myogenesis and Regulation of the Skeletal Muscle Glycogen Content in Mice
- Glycogen Contents in Skeletal Muscles in Men and Different Species of Experimental Animals
- MondoA Is Required for Normal Myogenesis and Regulation of the Skeletal Muscle Glycogen Content in Mice
- The Roles of Initial Level of Glycogen Content in Muscle and of Available Substrate on Muscle Glycogen Repletion in Rats
- Skeletal Muscle Glycogen Synthase in the Pathogenesis of Insulin Resistance