Clin Endosc.
2021 May;54(3):324-328. 10.5946/ce.2020.061.
Diagnosing Gastric Mesenchymal Tumors by Digital Endoscopic Ultrasonography Image Analysis
- Affiliations
-
- 1Department of Internal Medicine, Pusan National University School of Medicine and Biomedical Research Institute, Pusan National University Hospital, Busan, Korea
- KMID: 2516311
- DOI: http://doi.org/10.5946/ce.2020.061
Abstract
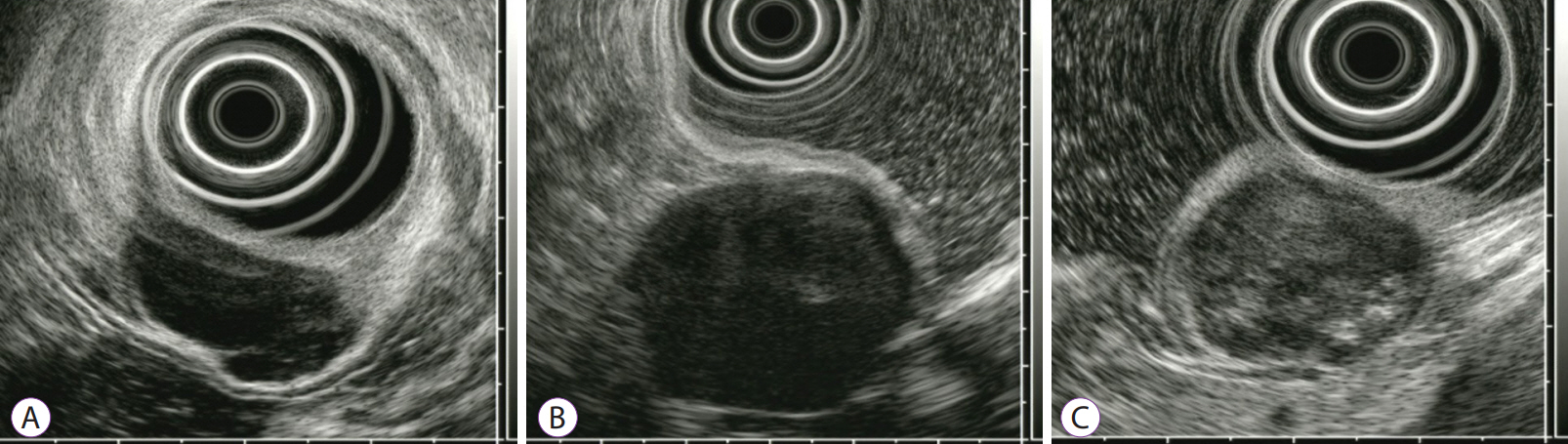
- Gastric mesenchymal tumors (GMTs) are incidentally discovered in national gastric screening programs in Korea. Endoscopic ultrasonography (EUS) is the most useful diagnostic modality for evaluating GMTs. The differentiation of gastrointestinal stromal tumors from benign mesenchymal tumors, such as schwannomas or leiomyomas, is important to ensure appropriate clinical management. However, this is difficult and operator dependent because of the subjective interpretation of EUS images. Digital image analysis computes the distribution and spatial variation of pixels using texture analysis to extract useful data, enabling the objective analysis of EUS images and decreasing interobserver and intraobserver agreement in EUS image interpretation. This review aimed to summarize the usefulness and future of digital EUS image analysis for GMTs based on published reports and our experience.
Figure
Reference
-
1. Pidhorecky I, Cheney RT, Kraybill WG, Gibbs JF. Gastrointestinal stromal tumors: current diagnosis, biologic behavior, and management. Ann Surg Oncol. 2000; 7:705–712.
Article2. Miettinen M, Sobin LH, Sarlomo-Rikala M. Immunohistochemical spectrum of GISTs at different sites and their differential diagnosis with a reference to CD117 (KIT). Mod Pathol. 2000; 13:1134–1142.
Article3. Miettinen M, Sobin LH, Lasota J. Gastrointestinal stromal tumors of the stomach: a clinicopathologic, immunohistochemical, and molecular genetic study of 1765 cases with long-term follow-up. Am J Surg Pathol. 2005; 29:52–68.4. Blay JY, Bonvalot S, Casali P, et al. Consensus meeting for the management of gastrointestinal stromal tumors. Report of the GIST consensus conference of 20-21 March 2004, under the auspices of ESMO. Ann Oncol. 2005; 16:566–578.
Article5. Fletcher CD, Berman JJ, Corless C, et al. Diagnosis of gastrointestinal stromal tumors: a consensus approach. Int J Surg Pathol. 2002; 10:81–89.
Article6. Demetri GD, von Mehren M, Antonescu CR, et al. NCCN task force report: update on the management of patients with gastrointestinal stromal tumors. J Natl Compr Canc Netw. 2010; 8(Suppl 2):S1–S41. quiz S42-S44.
Article7. Chak A, Canto MI, Rösch T, et al. Endosonographic differentiation of benign and malignant stromal cell tumors. Gastrointest Endosc. 1997; 45:468–473.
Article8. Palazzo L, Landi B, Cellier C, Cuillerier E, Roseau G, Barbier JP. Endosonographic features predictive of benign and malignant gastrointestinal stromal cell tumours. Gut. 2000; 46:88–92.
Article9. Kim GH, Park DY, Kim S, et al. Is it possible to differentiate gastric GISTs from gastric leiomyomas by EUS? World J Gastroenterol. 2009; 15:3376–3381.
Article10. Okai T, Minamoto T, Ohtsubo K, et al. Endosonographic evaluation of c-kit-positive gastrointestinal stromal tumor. Abdom Imaging. 2003; 28:301–307.
Article11. Catalano MF, Sivak MV Jr, Bedford RA, et al. Observer variation and reproducibility of endoscopic ultrasonography. Gastrointest Endosc. 1995; 41:115–120.
Article12. Gress F, Schmitt C, Savides T, et al. Interobserver agreement for EUS in the evaluation and diagnosis of submucosal masses. Gastrointest Endosc. 2001; 53:71–76.
Article13. Nishida T, Blay JY, Hirota S, Kitagawa Y, Kang YK. The standard diagnosis, treatment, and follow-up of gastrointestinal stromal tumors based on guidelines. Gastric Cancer. 2016; 19:3–14.
Article14. Min YW, Park HN, Min BH, Choi D, Kim KM, Kim S. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg. 2015; 19:631–638.
Article15. Yamada Y, Kida M, Sakaguchi T, et al. A study on myogenic tumors of the upper gastrointestinal tract by endoscopic ultrasonography-with special reference to the differential diagnosis of benign and malignant lesions. Dig Endosc. 1992; 4:396–408.
Article16. Yoon JM, Kim GH, Park DY, et al. Endosonographic features of gastric schwannoma: a single center experience. Clin Endosc. 2016; 49:548–554.
Article17. Tao K, Chang W, Zhao E, et al. Clinicopathologic features of gastric schwannoma: 8-year experience at a single institution in China. Medicine (Baltimore). 2015; 94:e1970.18. Park HC, Son DJ, Oh HH, et al. Endoscopic ultrasonographic characteristics of gastric schwannoma distinguished from gastrointestinal stromal tumor. Korean J Gastroenterol. 2015; 65:21–26.
Article19. Seo SW, Hong SJ, Han JP, et al. Accuracy of a scoring system for the differential diagnosis of common gastric subepithelial tumors based on endoscopic ultrasonography. J Dig Dis. 2013; 14:647–653.
Article20. Shah P, Gao F, Edmundowicz SA, Azar RR, Early DS. Predicting malignant potential of gastrointestinal stromal tumors using endoscopic ultrasound. Dig Dis Sci. 2009; 54:1265–1269.
Article21. Lee MW, Kim GH, Kim KB, et al. Digital image analysis-based scoring system for endoscopic ultrasonography is useful in predicting gastrointestinal stromal tumors. Gastric Cancer. 2019; 22:980–987.
Article22. Kim GH, Kim KB, Lee SH, et al. Digital image analysis of endoscopic ultrasonography is helpful in diagnosing gastric mesenchymal tumors. BMC Gastroenterol. 2014; 14:7.
Article23. Kim GH, Cho YK, Kim EY, et al. Comparison of 22-gauge aspiration needle with 22-gauge biopsy needle in endoscopic ultrasonography-guided subepithelial tumor sampling. Scand J Gastroenterol. 2014; 49:347–354.
Article24. Han JP, Lee TH, Hong SJ, et al. EUS-guided FNA and FNB after onsite cytological evaluation in gastric subepithelial tumors. J Dig Dis. 2016; 17:582–587.
Article25. Zhang XC, Li QL, Yu YF, et al. Diagnostic efficacy of endoscopic ultrasound-guided needle sampling for upper gastrointestinal subepithelial lesions: a meta-analysis. Surg Endosc. 2016; 30:2431–2441.
Article26. Nguyen VX, Nguyen CC, Li B, Das A. Digital image analysis is a useful adjunct to endoscopic ultrasonographic diagnosis of subepithelial lesions of the gastrointestinal tract. J Ultrasound Med. 2010; 29:1345–1351.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gastric Schwannoma: A Case Report
- Gastric Subepithelial Tumor Diagnosed by Transabdominal Ultrasonography
- Endoscopic Characteristics of Upper Gastrointestinal Mesenchymal Tumors Originating from Muscularis Mucosa or Muscularis Propria
- A case of submucosal gastric lymphoepithelioma-like carcinoma
- Gastric Schwannoma Diagnosed by Endoscopic Ultrasonography-Guided Trucut Biopsy