J Korean Med Sci.
2021 May;36(21):e142. 10.3346/jkms.2021.36.e142.
Real-world Effectiveness and Safety of Direct-acting Antiviral Agents in Patients with Chronic Hepatitis C Genotype 2 Infection: Korean Multicenter Study
- Affiliations
-
- 1Department of Internal Medicine, Dong-A University College of Medicine, Busan, Korea
- 2Department of Internal Medicine, Inje University Busan Paik Hospital, Busan, Korea
- 3Department of Internal Medicine, Pusan National University Yangsan Hospital, Yangsan, Korea
- 4Department of Internal Medicine, Inje University Haeundae Paik Hospital, Busan, Korea
- 5Department of Internal Medicine, Kosin University Gospel Hospital, Busan, Korea
- 6Department of Internal Medicine, Gyeongsang National University School of Medicine and Gyeongsang National University Hospital, Jinju, Korea
- 7Department of Internal Medicine, College of Medicine, Gyeongsang National University, Jinju, Korea
- 8Department of Internal Medicine, Ulsan University College of Medicine, Ulsan, Korea
- KMID: 2516253
- DOI: http://doi.org/10.3346/jkms.2021.36.e142
Abstract
- Background
The advancement of treatment with direct-acting antiviral (DAA) agents has improved the cure rate of hepatitis C virus (HCV) infection close to 100%. The aim of our study was to assess the real-world effectiveness and safety of DAA regimens for the treatment of patients with chronic HCV genotype 2.
Methods
We retrospectively analyzed the clinical data of patients treated with sofosbuvir plus ribavirin (SOF + RBV) or glecaprevir/pibrentasvir (G/P) for chronic HCV genotype 2 infection at seven university hospitals in the Korean southeast region.
Results
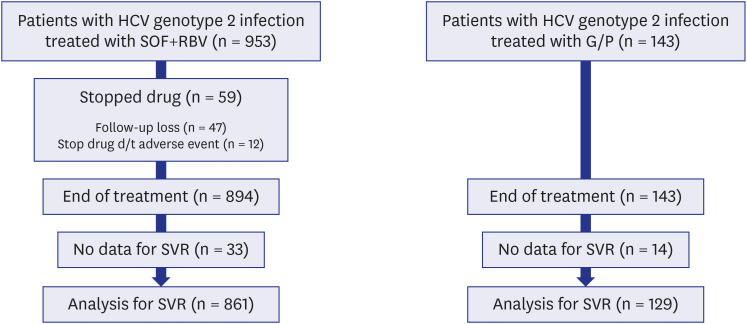
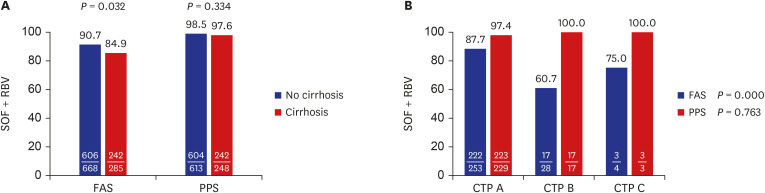
SOF + RBV therapy produced an 89% and 98.3% sustained virologic response 12 week (SVR12) after treatment completion in the full analysis set and per-protocol set, respectively, and the corresponding values for G/P therapy were 89.5% and 99.2%, respectively. The difference between the treatments was probably because 6.2% (59/953) of patients in the SOF + RBV group did not complete the treatment and 9.8% (14/143) in the G/P group did not test HCV RNA after treatment completion. Adverse events (A/Es) were reported in 59.7% (569/953) and 25.9% (37/143) of the SOF + RBV and G/P groups, respectively. In the SOF + RBV group, 12 (1.26%) patients discontinued treatment owing to A/Es, whereas no patients discontinued treatment because of A/Es in the G/P group.
Conclusion
In both treatment groups, SVR was high when treatment was completed. However, there was a high dropout rate in the SOF + RBV group, and the dropout analysis showed that these were patients with liver cirrhosis (LC; 43/285, 15.1%), especially those with decompensated LC (12/32, 37.5%). Therefore, an early initiation of antiviral therapy is recommended for a successful outcome before liver function declines. Furthermore, patients with decompensated LC who are considered candidates for SOF + RBV treatment should be carefully monitored to ensure that their treatment is completed, especially those with low hemoglobin and high alanine transaminase.
Figure
Reference
-
1. Kao JH. Hepatitis C virus infection in Taiwan: past, present, and future. J Formos Med Assoc. 2016; 115(2):65–66. PMID: 26228687.
Article2. Liu CH, Kao JH. Nanomedicines in the treatment of hepatitis C virus infection in Asian patients: optimizing use of peginterferon alfa. Int J Nanomedicine. 2014; 9:2051–2067. PMID: 24812506.3. European Association for the Study of the Liver. EASL recommendations on treatment of hepatitis C 2018. J Hepatol. 2018; 69(2):461–511. PMID: 29650333.4. European Association for Study of Liver. EASL clinical practice guidelines: management of hepatitis C virus infection. J Hepatol. 2014; 60(2):392–420. PMID: 24331294.5. Prenner SB, VanWagner LB, Flamm SL, Salem R, Lewandowski RJ, Kulik L. Hepatocellular carcinoma decreases the chance of successful hepatitis C virus therapy with direct-acting antivirals. J Hepatol. 2017; 66(6):1173–1181. PMID: 28161470.
Article6. Kanda T, Imazeki F, Yokosuka O. New antiviral therapies for chronic hepatitis C. Hepatol Int. 2010; 4(3):548–561. PMID: 21063477.
Article7. von Wagner M, Huber M, Berg T, Hinrichsen H, Rasenack J, Heintges T, et al. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005; 129(2):522–527. PMID: 16083709.8. Sato K, Hashizume H, Yamazaki Y, Horiguchi N, Kakizaki S, Takagi H, et al. Response-guided peginterferon-alpha-2b plus ribavirin therapy for chronic hepatitis C patients with genotype 2 and high viral loads. Hepatol Res. 2012; 42(9):854–863. PMID: 22487210.
Article9. Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: an open-label, phase 3 trial. J Viral Hepat. 2014; 21(11):762–768. PMID: 25196837.10. Kao JH, Chien RN, Chang TT, Peng CY, Hu TH, Lo GH, et al. A phase 3b study of sofosbuvir plus ribavirin in Taiwanese patients with chronic genotype 2 hepatitis C virus infection. Liver Int. 2016; 36(8):1101–1107. PMID: 26835876.
Article11. Kwo PY, Poordad F, Asatryan A, Wang S, Wyles DL, Hassanein T, et al. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J Hepatol. 2017; 67(2):263–271. PMID: 28412293.
Article12. Asselah T, Kowdley KV, Zadeikis N, Wang S, Hassanein T, Horsmans Y, et al. Efficacy of glecaprevir/pibrentasvir for 8 or 12 weeks in patients with hepatitis C virus genotype 2, 4, 5, or 6 infection without cirrhosis. Clin Gastroenterol Hepatol. 2018; 16(3):417–426. PMID: 28951228.
Article13. Forns X, Lee SS, Valdes J, Lens S, Ghalib R, Aguilar H, et al. Glecaprevir plus pibrentasvir for chronic hepatitis C virus genotype 1, 2, 4, 5, or 6 infection in adults with compensated cirrhosis (EXPEDITION-1): a single-arm, open-label, multicentre phase 3 trial. Lancet Infect Dis. 2017; 17(10):1062–1068. PMID: 28818546.
Article14. Puoti M, Foster GR, Wang S, Mutimer D, Gane E, Moreno C, et al. High SVR12 with 8-week and 12-week glecaprevir/pibrentasvir therapy: an integrated analysis of HCV genotype 1-6 patients without cirrhosis. J Hepatol. 2018; 69(2):293–300. PMID: 29551706.
Article15. van der Meer AJ, Veldt BJ, Feld JJ, Wedemeyer H, Dufour JF, Lammert F, et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA. 2012; 308(24):2584–2593. PMID: 23268517.
Article16. Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017; S0168-8278(17)32273-0.
Article17. Ghany MG, Morgan TR. AASLD-IDSA Hepatitis C Guidance Panel. Hepatitis C guidance 2019 update: American Association for the Study of Liver Diseases-Infectious Diseases Society of America recommendations for testing, managing, and treating hepatitis C virus infection. Hepatology. 2020; 71(2):686–721. PMID: 31816111.
Article18. Yeon JE. Recent update of the 2017 Korean Association for the Study of the Liver (KASL) treatment guidelines of chronic hepatitis C: comparison of guidelines from other continents, 2017 AASLD/IDSA and 2016 EASL. Clin Mol Hepatol. 2018; 24(3):278–293. PMID: 29716179.
Article19. Jung YK. Renewed 2015 clinical practice guidelines for management of hepatitis C by Korean Association for the Study of the Liver; What has been changed? - treatment of chronic hepatitis C genotype 2 and 3. Korean J Gastroenterol. 2016; 67(3):132–136. PMID: 26996182.
Article20. Seong MH, Kil H, Kim YS, Bae SH, Lee YJ, Lee HC, et al. Clinical and epidemiological features of hepatitis C virus infection in South Korea: a prospective, multicenter cohort study. J Med Virol. 2013; 85(10):1724–1733. PMID: 23813472.
Article21. Kim DY, Kim IH, Jeong SH, Cho YK, Lee JH, Jin YJ, et al. A nationwide seroepidemiology of hepatitis C virus infection in South Korea. Liver Int. 2013; 33(4):586–594. PMID: 23356674.
Article22. Kim MN, Kim BK, Han KH. Hepatocellular carcinoma in patients with chronic hepatitis C virus infection in the Asia-Pacific region. J Gastroenterol. 2013; 48(6):681–688. PMID: 23463401.
Article23. Akahane T, Kurosaki M, Itakura J, Tsuji K, Joko K, Kimura H, et al. Real-world efficacy and safety of sofosbuvir + ribavirin for hepatitis C genotype 2: a nationwide multicenter study by the Japanese Red Cross Liver Study Group. Hepatol Res. 2019; 49(3):264–270. PMID: 30171740.
Article24. Verna EC, Morelli G, Terrault NA, Lok AS, Lim JK, Di Bisceglie AM, et al. DAA therapy and long-term hepatic function in advanced/decompensated cirrhosis: Real-world experience from HCV-TARGET cohort. J Hepatol. 2020; 73(3):540–548. PMID: 32243960.
Article25. Chua JV, Kottilil S. Sofosbuvir and velpatasvir: a stellar option for patients with decompensated hepatitis C virus (HCV) cirrhosis. Ann Transl Med. 2016; 4(Suppl 1):S8. PMID: 27867976.
Article26. Feld JJ, Jacobson IM, Hézode C, Asselah T, Ruane PJ, Gruener N, et al. Sofosbuvir and velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 infection. N Engl J Med. 2015; 373(27):2599–2607. PMID: 26571066.
Article27. D'Ambrosio R, Pasulo L, Puoti M, Vinci M, Schiavini M, Lazzaroni S, et al. Real-world effectiveness and safety of glecaprevir/pibrentasvir in 723 patients with chronic hepatitis C. J Hepatol. 2019; 70(3):379–387. PMID: 30472321.28. Ikeda H, Watanabe T, Atsukawa M, Toyoda H, Takaguchi K, Nakamuta M, et al. Evaluation of 8-week glecaprevir/pibrentasvir treatment in direct-acting antiviral-naïve noncirrhotic HCV genotype 1 and 2infected patients in a real-world setting in Japan. J Viral Hepat. 2019; 26(11):1266–1275. PMID: 31278795.
Article29. Ji F, Yeo YH, Wei MT, Ogawa E, Enomoto M, Lee DH, et al. Sustained virologic response to direct-acting antiviral therapy in patients with chronic hepatitis C and hepatocellular carcinoma: a systematic review and meta-analysis. J Hepatol. 2019; 71(3):473–485. PMID: 31096005.
Article30. Wirth TC, Manns MP. The impact of the revolution in hepatitis C treatment on hepatocellular carcinoma. Ann Oncol. 2016; 27(8):1467–1474. PMID: 27226385.
Article31. Hengst J, Schlaphoff V, Deterding K, Falk C, Manns M, Cornberg M, et al. DAA-induced HCV clearance does not restore the altered cytokine and chemokine milieu in patients with chronic hepatitis C. J Hepatol. 2016; 64(2):S417–S418.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Assessing the Effectiveness and Safety of Direct-acting Antiviral Treatment in Korean Patients with Hepatitis C Virus Genotype 1b or 2 at a Tertiary Care Hospital
- 2017 Korean Association for the Study of the Liver (KASL) Clinical Practice Guidelines of Chronic Hepatitis C: What's New?
- Management of hepatitis C viral infection in chronic kidney disease patients on hemodialysis in the era of direct-acting antivirals
- Recent update of the 2017 Korean Association for the Study of the Liver (KASL) treatment guidelines of chronic hepatitis C: Comparison of guidelines from other continents, 2017 AASLD/IDSA and 2016 EASL
- Treatment of Chronic Hepatitis C Patients Who Do Not Respond to Direct Acting Antivirals