Ann Surg Treat Res.
2021 Jun;100(6):305-312. 10.4174/astr.2021.100.6.305.
Targeted axillary biopsy and sentinel lymph node biopsy for axillary restaging after neoadjuvant chemotherapy
- Affiliations
-
- 1Department of Surgery, Health Science University, Haydarpasa Numune Research and Training Hospital, Istanbul, Turkey
- 2Department of Radiology, Health Science University, Haydarpasa Numune Research and Training Hospital, Istanbul, Turkey
- 3Department of Pathology, Health Science University, Haydarpasa Numune Research and Training Hospital, Istanbul, Turkey
- 4Department of Medical Oncology, Health Science University, Haydarpasa Numune Research and Training Hospital, Istanbul, Turkey
- 5Department of Surgery, Duzce University Medical Faculty, Duzce, Turkey
- KMID: 2516223
- DOI: http://doi.org/10.4174/astr.2021.100.6.305
Abstract
- Purpose
Accurate restaging of the axilla after neoadjuvant chemotherapy (NAC) is an important issue to ensure deescalating axillary surgery in patients with initial metastatic nodes. We aimed to present our results of targeted axillary biopsy (TAB) combined with sentinel lymph node biopsy (SLNB) for axillary restaging after NAC.
Methods
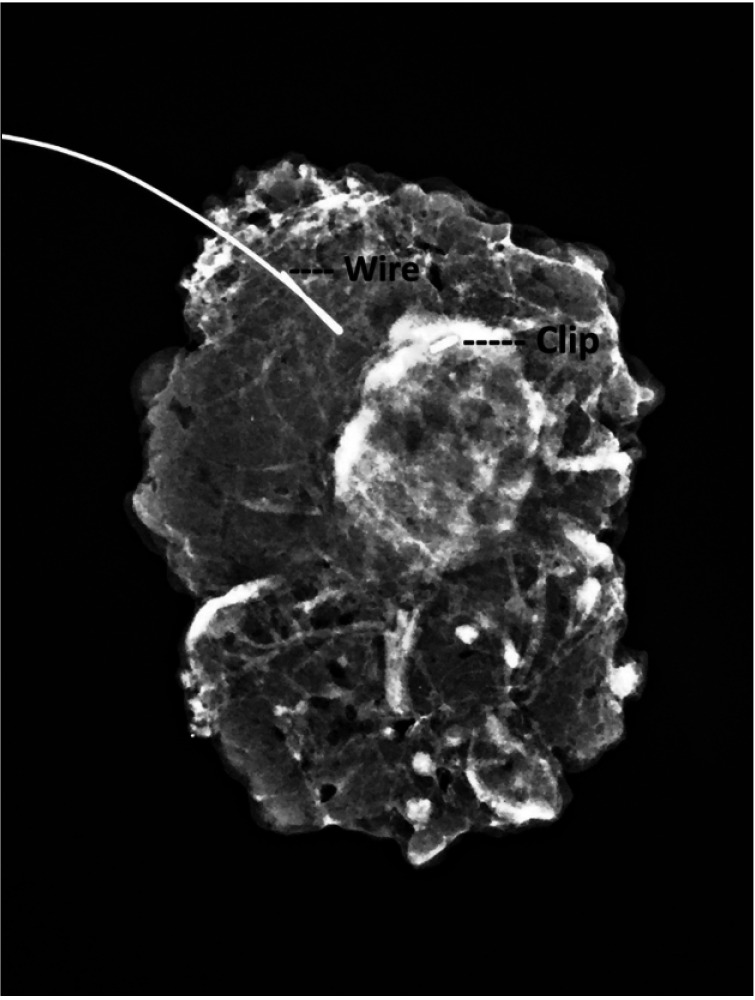
In 64 breast cancer patients who underwent NAC, biopsy-proven positive nodes were marked with clips before NAC, and ultrasound-guided wire localization of clip-marked nodes was performed after NAC. Patients underwent TAB and SLNB for post-NAC axilla restaging.
Results
Identification rates of post-NAC TAB and SLNB were 98.4% and 87.5%, respectively (P = 0.033). Histopathology revealed a nodal pathologic complete response (pCR) rate of 47% in which axillary lymph node dissection (ALND) was avoided. TAB alone and SLNB alone detected residual disease in 29 (85.3%) and 20 (58.8%) patients (P = 0.029), respectively. Whereas rates of up to 97% had been achieved with a combination of TAB and SLNB. The pCR rates after NAC were 64.3% for human epidermal growth factor receptor 2 positive and triple-negative tumors and 13.6% in luminal tumors (P = 0.0002).
Conclusion
Pathologic analysis following TAB combined with SLNB revealed the pCR rates to NAC in a considerable number of patients that provided de-escalation of axillary surgery. A combination of SLNB and TAB was found to be an accurate procedure in establishing residual nodal disease. This combined procedure in patients with initially positive nodes was a reliable method for post-NAC axillary restaging.
Keyword
Figure
Reference
-
1. Wong SM, Weiss A, Mittendorf EA, King TA, Golshan M. Surgical management of the axilla in clinically node-positive patients receiving neoadjuvant chemotherapy: a national cancer database analysis. Ann Surg Oncol. 2019; 26:3517–3525. PMID: 31342389.
Article2. Kim JY, Kim MK, Lee JE, Jung Y, Bae SY, Lee SK, et al. Sentinel lymph node biopsy alone after neoadjuvant chemotherapy in patients with initial cytology-proven axillary node metastasis. J Breast Cancer. 2015; 18:22–28. PMID: 25834607.
Article3. Simons JM, van Nijnatten TJ, van der Pol CC, Luiten EJ, Koppert LB, Smidt ML. Diagnostic accuracy of different surgical procedures for axillary staging after neoadjuvant systemic therapy in node-positive breast cancer: a systematic review and meta-analysis. Ann Surg. 2019; 269:432–442. PMID: 30312200.4. Dashevsky BZ, Altman A, Abe H, Jaskowiak N, Bao J, Schacht DV, et al. Lymph node wire localization post-chemotherapy: towards improving the false negative sentinel lymph node biopsy rate in breast cancer patients. Clin Imaging. 2018; 48:69–73. PMID: 29035756.
Article5. Diego EJ, McAuliffe PF, Soran A, McGuire KP, Johnson RR, Bonaventura M, et al. Axillary staging after neoadjuvant chemotherapy for breast cancer: a pilot study combining sentinel lymph node biopsy with radioactive seed localization of pre-treatment positive axillary lymph nodes. Ann Surg Oncol. 2016; 23:1549–1553. PMID: 26727919.
Article6. Racz JM, Caudle AS. Sentinel node lymph node surgery after neoadjuvant therapy: principles and techniques. Ann Surg Oncol. 2019; 26:3040–3045. PMID: 31342394.
Article7. Hartmann S, Reimer T, Gerber B, Stubert J, Stengel B, Stachs A. Wire localization of clip-marked axillary lymph nodes in breast cancer patients treated with primary systemic therapy. Eur J Surg Oncol. 2018; 44:1307–1311. PMID: 29935839.
Article8. Sutton TL, Johnson N, Garreau JR. Adequate sentinel node harvest is associated with low false negative rate in breast cancer managed with neoadjuvant chemotherapy and targeted axillary dissection. Am J Surg. 2020; 219:851–854. PMID: 32245609.
Article9. Park S, Koo JS, Kim GM, Sohn J, Kim SI, Cho YU, et al. Feasibility of charcoal tattooing of cytology-proven metastatic axillary lymph node at diagnosis and sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. Cancer Res Treat. 2018; 50:801–812. PMID: 28814071.
Article10. Choi HJ, Kim I, Alsharif E, Park S, Kim JM, Ryu JM, et al. Use of sentinel lymph node biopsy after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer in diagnosis. J Breast Cancer. 2018; 21:433–441. PMID: 30607165.
Article11. Spautz CC, Schunemann Junior E, Budel LR, Cavalcanti TC, Louveira MH, Junior PG, et al. Marking axillary nodes with 4% carbon microparticle suspension before neoadjuvant chemotherapy improves sentinel node identification rate and axillary staging. J Surg Oncol. 2020; 122:164–169. PMID: 32291774.
Article12. Kim WH, Kim HJ, Kim SH, Jung JH, Park HY, Lee J, et al. Ultrasound-guided dual-localization for axillary nodes before and after neoadjuvant chemotherapy with clip and activated charcoal in breast cancer patients: a feasibility study. BMC Cancer. 2019; 19:859. PMID: 31470821.
Article13. Jung SY, Han JH, Park SJ, Lee EG, Kwak J, Kim SH, et al. The sentinel lymph node biopsy using indocyanine green fluorescence plus radioisotope method compared with the radioisotope-only method for breast cancer patients after neoadjuvant chemotherapy: a prospective, randomized, open-label, single-center phase 2 trial. Ann Surg Oncol. 2019; 26:2409–2416. PMID: 31065958.
Article14. Kim HS, Shin MS, Kim CJ, Yoo SH, Yoo TK, Eom YH, et al. Improved model for predicting axillary response to neoadjuvant chemotherapy in patients with clinically node-positive breast cancer. J Breast Cancer. 2017; 20:378–385. PMID: 29285043.
Article15. Morency D, Dumitra S, Parvez E, Martel K, Basik M, Robidoux A, et al. Axillary lymph node ultrasound fol lowing neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: results from the SN FNAC Study. Ann Surg Oncol. 2019; 26:4337–4345. PMID: 31605348.16. Plecha D, Bai S, Patterson H, Thompson C, Shenk R. Improving the accuracy of axillar y lymph node surger y in breast cancer with ultrasound-guided wire localization of biopsy proven metastatic lymph nodes. Ann Surg Oncol. 2015; 22:4241–4246. PMID: 25814365.17. Cabıoğlu N, Karanlık H, Kangal D, Özkurt E, Öner G, Sezen F, et al. Improved false-negative rates with intraoperative identification of clipped nodes in patients undergoing sentinel lymph node biopsy after neoadjuvant chemotherapy. Ann Surg Oncol. 2018; 25:3030–3036. PMID: 29978371.
Article18. Laws A, Dillon K, Kelly BN, Kantor O, Hughes KS, Gadd MA, et al. Node-positive patients treated with neoadjuvant chemotherapy can be spared axillary lymph node dissection with wireless non-radioactive localizers. Ann Surg Oncol. 2020; 27:4819–4827. PMID: 32740737.
Article19. Han A, Moon HG, Kim J, Ahn SK, Park IA, Han W, et al. Reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients. J Breast Cancer. 2013; 16:378–385. PMID: 24454459.
Article20. Kim WH, Kim HJ, Park CS, Lee J, Park HY, Jung JH, et al. Axillary nodal burden assessed with pretreatment breast MRI is associated with failed sentinel lymph node identification after neoadjuvant chemotherapy for breast cancer. Radiology. 2020; 295:275–282. PMID: 32125253.21. Kim EY, Byon WS, Lee KH, Yun JS, Park YL, Park CH, et al. Feasibility of preoperative axillary lymph node marking with a clip in breast cancer patients before neoadjuvant chemotherapy: a preliminary study. World J Surg. 2018; 42:582–589. PMID: 28808843.
Article22. Balasubramanian R, Morgan C, Shaari E, Kovacs T, Pinder SE, Hamed H, et al. Wire guided localisation for targeted axillary node dissection is accurate in axillary staging in node positive breast cancer following neoadjuvant chemotherapy. Eur J Surg Oncol. 2020; 46:1028–1033. PMID: 31879050.
Article23. Kanesalingam K, Sriram N, Heilat G, Ng EE, Meybodi F, Elder E, et al. Targeted axillary dissection after neoadjuvant systemic therapy in patients with nodepositive breast cancer. ANZ J Surg. 2020; 90:332–338. PMID: 31845501.
Article24. Simons JM, van Pelt ML, Marinelli AW, Straver ME, Zeillemaker AM, Pereira Arias-Bouda LM, et al. Excision of both pretreatment marked positive nodes and sentinel nodes improves axillary staging after neoadjuvant systemic therapy in breast cancer. Br J Surg. 2019; 106:1632–1639. PMID: 31593294.
Article25. Al-Hattali S, Vinnicombe SJ, Gowdh NM, Evans A, Armstrong S, Adamson D, et al. Breast MRI and tumour biology predict axillary lymph node response to neoadjuvant chemotherapy for breast cancer. Cancer Imaging. 2019; 19:91. PMID: 31878958.
Article26. Lim GH, Gudi M, Teo SY, Ng RP, Yan Z, Lee YS, et al. Would removal of all ultrasound abnormal metastatic lymph nodes without sentinel lymph node biopsy be accurate in patients with breast cancer with neoadjuvant chemotherapy. Oncologist. 2020; 25:e1621–e1627. PMID: 32537791.
Article27. Choi HJ, Ryu JM, Kim I, Nam SJ, Kim SW, Yu J, et al. Nomogram for accurate prediction of breast and axillary pathologic response after neoadjuvant chemotherapy in node positive patients with breast cancer. Ann Surg Treat Res. 2019; 96:169–176. PMID: 30941320.
Article28. Simons JM, Koppert LB, Luiten EJ, van der Pol CC, Samiei S, de Wilt JH, et al. Deescalation of axillary surgery in breast cancer patients treated in the neoadjuvant setting: a Dutch population-based study. Breast Cancer Res Treat. 2020; 180:725–733. PMID: 32180074.
Article29. Caudle AS, Yang WT, Krishnamurthy S, Mittendorf EA, Black DM, Gilcrease MZ, et al. Improved axillary evaluation following neoadjuvant therapy for patients with node-positive breast cancer using selective evaluation of clipped nodes: implementation of targeted axillary dissection. J Clin Oncol. 2016; 34:1072–1078. PMID: 26811528.
Article30. Nguyen TT, Hieken TJ, Glazebrook KN, Boughey JC. Localizing the clipped node in patients with node-positive breast cancer treated with neoadjuvant chemotherapy: early learning experience and chal lenges. Ann Surg Oncol. 2017; 24:3011–3016. PMID: 28766234.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Evaluation of the Role of Axillary Lymph Node Fine-Needle Aspiration Cytology in Early Breast Cancer With or Without Neoadjuvant Chemotherapy
- Effectiveness of Sentinel Node Biopsy in the Prediction of Axillary Nodal Status in 111 Patients with Breast Cancer
- Prospective Evaluation of the Feasibility of Sentinel Lymph Node Biopsy in Breast Cancer Patients with Negative Axillary Conversion after Neoadjuvant Chemotherapy
- Effectiveness of Sentinel Node Biopsy in the Prediction of Axillary Nodal Status in 111 Patients with Breast Cancer
- Analysis of Sentinel Lymph Node Biopsy in 500 Cases with Breast Cancer