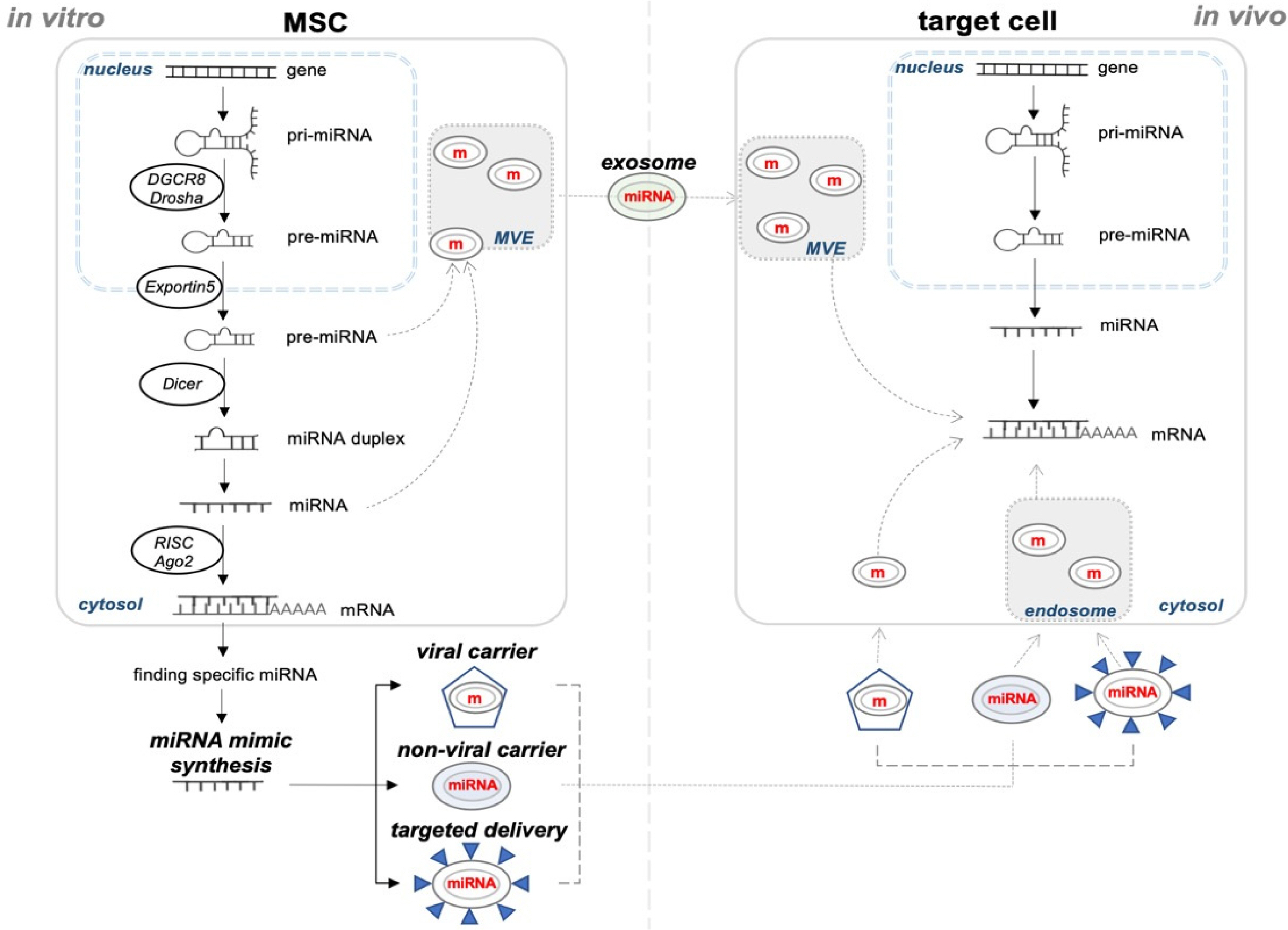
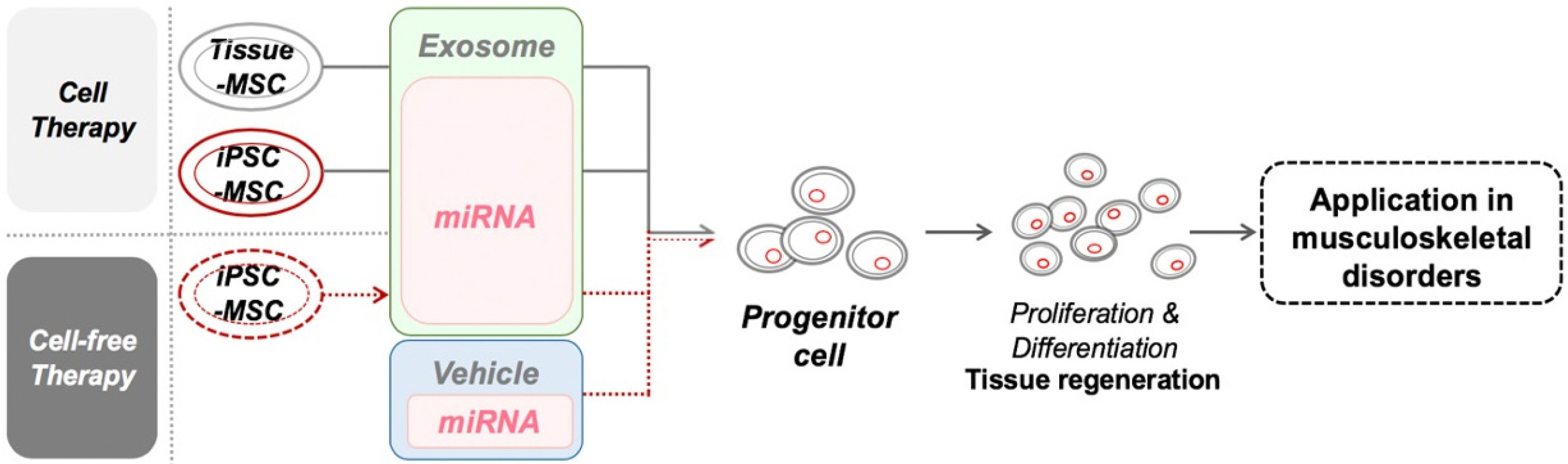
Int J Stem Cells.
2021 May;14(2):150-167. 10.15283/ijsc20167.
Mesenchymal Stem Cell and MicroRNA Therapy of Musculoskeletal Diseases
- Affiliations
-
- 1Department of Pathology, College of Veterinary Medicine, Kyungpook National University, Daegu, Korea
- 2Stem Cell Therapeutic Research Institute, Kyungpook National University, Daegu, Korea
- 3School of Medicine, Kyungpook National University, Daegu, Korea
- 4Department of Food Science & Biotechnology, Kyungpook National University, Daegu, Korea
- KMID: 2515992
- DOI: http://doi.org/10.15283/ijsc20167
Abstract
- The therapeutic effects of mesenchymal stem cells (MSCs) in musculoskeletal diseases (MSDs) have been verified in many human and animal studies. Although some tissues contain MSCs, the number of cells harvested from those tissues and rate of proliferation in vitro are not enough for continuous transplantation. In order to produce and maintain stable MSCs, many attempts are made to induce differentiation from pluripotent stem cells (iPSCs) into MSCs. In particular, it is also known that the paracrine action of stem cell-secreted factors could promote the regeneration and differentiation of target cells in damaged tissue. MicroRNAs (miRNAs), one of the secreted factors, are small non-coding RNAs that regulate the translation of a gene. It is known that miRNAs help communication between stem cells and their surrounding niches through exosomes to regulate the proliferation and differentiation of stem cells. While studies have so far been underway targeting therapeutic miRNAs of MSDs, studies on specific miRNAs secreted from MSCs are still minimal. Hence, our ultimate goal is to obtain sufficient amounts of exosomes from iPSC-MSCs and develop them into therapeutic agents, furthermore to select specific miRNAs and provide safe cell-free clinical setting as a cell-free status with purpose of delivering them to target cells. This review article focuses on stem cell therapy on MSDs, specific microRNAs regulating MSDs and updates on novel approaches.
Keyword
Figure
Reference
-
References
1. Bongers PM, de Winter CR, Kompier MA, Hildebrandt VH. 1993; Psychosocial factors at work and musculoskeletal disease. Scand J Work Environ Health. 19:297–312. DOI: 10.5271/sjweh.1470. PMID: 8296178.
Article2. Barbe MF, Gallagher S, Massicotte VS, Tytell M, Popoff SN, Barr-Gillespie AE. 2013; The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet Disord. 14:303. DOI: 10.1186/1471-2474-14-303. PMID: 24156755. PMCID: PMC3924406.
Article3. Bianco P, Robey PG, Simmons PJ. 2008; Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell. 2:313–319. DOI: 10.1016/j.stem.2008.03.002. PMID: 18397751. PMCID: PMC2613570.
Article4. Lu D, Chen B, Liang Z, Deng W, Jiang Y, Li S, Xu J, Wu Q, Zhang Z, Xie B, Chen S. 2011; Comparison of bone marrow mesenchymal stem cells with bone marrow-derived mononuclear cells for treatment of diabetic critical limb ischemia and foot ulcer: a double-blind, randomized, controlled trial. Diabetes Res Clin Pract. 92:26–36. DOI: 10.1016/j.diabres.2010.12.010. PMID: 21216483.
Article5. Yamada Y, Ueda M, Hibi H, Baba S. 2006; A novel approach to periodontal tissue regeneration with mesenchymal stem cells and platelet-rich plasma using tissue engineering technology: a clinical case report. Int J Periodontics Restorative Dent. 26:363–369. PMID: 16939018.6. Böcker W, Yin Z, Drosse I, Haasters F, Rossmann O, Wierer M, Popov C, Locher M, Mutschler W, Docheva D, Schieker M. 2008; Introducing a single-cell-derived human mesenchymal stem cell line expressing hTERT after lentiviral gene transfer. J Cell Mol Med. 12:1347–1359. DOI: 10.1111/j.1582-4934.2008.00299.x. PMID: 18318690. PMCID: PMC3865677.
Article7. Stolzing A, Jones E, McGonagle D, Scutt A. 2008; Age-related changes in human bone marrow-derived mesenchymal stem cells: consequences for cell therapies. Mech Ageing Dev. 129:163–173. DOI: 10.1016/j.mad.2007.12.002. PMID: 18241911.
Article8. Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. 2008; Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 7:335–343. DOI: 10.1111/j.1474-9726.2008.00377.x. PMID: 18248663. PMCID: PMC2398731.
Article9. Whitworth DJ, Banks TA. 2014; Stem cell therapies for treating osteoarthritis: prescient or premature? Vet J. 202:416–424. DOI: 10.1016/j.tvjl.2014.09.024. PMID: 25457267.
Article10. Chung MJ, Park S, Son JY, Lee JY, Yun HH, Lee EJ, Lee EM, Cho GJ, Lee S, Park HS, Jeong KS. 2019; Differentiation of equine induced pluripotent stem cells into mesenchymal lineage for therapeutic use. Cell Cycle. 18:2954–2971. DOI: 10.1080/15384101.2019.1664224. PMID: 31505996. PMCID: PMC6791704.
Article11. Lian Q, Zhang Y, Zhang J, Zhang HK, Wu X, Zhang Y, Lam FF, Kang S, Xia JC, Lai WH, Au KW, Chow YY, Siu CW, Lee CN, Tse HF. 2010; Functional mesenchymal stem cells derived from human induced pluripotent stem cells attenuate limb ischemia in mice. Circulation. 121:1113–1123. DOI: 10.1161/CIRCULATIONAHA.109.898312. PMID: 20176987.
Article12. Mehta G, Shiozawa Y, Taichman R. Ma P, editor. 2014. Hematopoietic stem cells and their niches. Biomaterials and Regenerative Medicine. Cambridge University Press;Cambridge: p. 44–63. DOI: 10.1017/CBO9780511997839.006. PMCID: PMC3636104.
Article13. Wang W, Zhang E, Lin C. 2015; MicroRNAs in tumor angio-genesis. Life Sci. 136:28–35. DOI: 10.1016/j.lfs.2015.06.025. PMID: 26144623.
Article14. Calin GA, Croce CM. 2006; MicroRNA signatures in human cancers. Nat Rev Cancer. 6:857–866. DOI: 10.1038/nrc1997. PMID: 17060945.
Article15. Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, Parenti AR, Daidone MG, Bicciato S, Piccolo S. 2010; A microRNA targeting dicer for metastasis control. Cell. 141:1195–1207. DOI: 10.1016/j.cell.2010.05.017. PMID: 20603000.
Article16. Xie T, Huang M, Wang Y, Wang L, Chen C, Chu X. 2016; MicroRNAs as regulators, biomarkers and therapeutic targets in the drug resistance of colorectal cancer. Cell Physiol Biochem. 40:62–76. DOI: 10.1159/000452525. PMID: 27842308.
Article17. Ambros V. 2004; The functions of animal microRNAs. Nature. 431:350–355. DOI: 10.1038/nature02871. PMID: 15372042.
Article18. Bartel DP. 2004; MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 116:281–297. DOI: 10.1016/S0092-8674(04)00045-5. PMID: 14744438.19. Bartel DP. 2018; Metazoan microRNAs. Cell. 173:20–51. DOI: 10.1016/j.cell.2018.03.006. PMID: 29570994. PMCID: PMC6091663.
Article20. van Niel G, D'Angelo G, Raposo G. 2018; Shedding light on the cell biology of extracellular vesicles. Nat Rev Mol Cell Biol. 19:213–228. DOI: 10.1038/nrm.2017.125. PMID: 29339798.
Article21. Phan J, Kumar P, Hao D, Gao K, Farmer D, Wang A. 2018; Engineering mesenchymal stem cells to improve their exosome efficacy and yield for cell-free therapy. J Extracell Vesicles. 7:1522236. DOI: 10.1080/20013078.2018.1522236. PMID: 30275938. PMCID: PMC6161586.
Article22. Rani S, Ryan AE, Griffin MD, Ritter T. 2015; Mesenchymal stem cell-derived extracellular vesicles: toward cell-free therapeutic applications. Mol Ther. 23:812–823. DOI: 10.1038/mt.2015.44. PMID: 25868399. PMCID: PMC4427881.
Article23. Pinheiro A, Silva AM, Teixeira JH, Gonçalves RM, Almeida MI, Barbosa MA, Santos SG. 2018; Extracellular vesicles: intelligent delivery strategies for therapeutic applications. J Control Release. 289:56–69. DOI: 10.1016/j.jconrel.2018.09.019. PMID: 30261205.
Article24. Lo Cicero A, Stahl PD, Raposo G. 2015; Extracellular vesicles shuffling intercellular messages: for good or for bad. Curr Opin Cell Biol. 35:69–77. DOI: 10.1016/j.ceb.2015.04.013. PMID: 26001269.
Article25. Chu DT, Phuong TNT, Tien NLB, Tran DK, Thanh VV, Quang TL, Truong DT, Pham VH, Ngoc VTN, Chu-Dinh T, Kushekhar K. 2020; An update on the progress of isolation, culture, storage, and clinical application of human bone marrow mesenchymal stem/stromal cells. Int J Mol Sci. 21:708. DOI: 10.3390/ijms21030708. PMID: 31973182. PMCID: PMC7037097.
Article26. Bunnell BA, Flaat M, Gagliardi C, Patel B, Ripoll C. 2008; Adipose-derived stem cells: isolation, expansion and diffe-rentiation. Methods. 45:115–120. DOI: 10.1016/j.ymeth.2008.03.006. PMID: 18593609. PMCID: PMC3668445.
Article27. Mazini L, Rochette L, Amine M, Malka G. 2019; Regenerative capacity of adipose derived stem cells (ADSCs), comparison with mesenchymal stem cells (MSCs). Int J Mol Sci. 20:2523. DOI: 10.3390/ijms20102523. PMID: 31121953. PMCID: PMC6566837.
Article28. Duckers HJ, Pinkernell K, Milstein AM, Hedrick MH. 2006; The Bedside Celution system for isolation of adipose derived regenerative cells. EuroIntervention. 2:395–398. PMID: 19755319.29. Coleman SR. 2006; Structural fat grafting: more than a permanent filler. Plast Reconstr Surg. 118(3 Suppl):108S–120S. DOI: 10.1097/01.prs.0000234610.81672.e7. PMID: 16936550.
Article30. Van RL, Roncari DA. 1977; Isolation of fat cell precursors from adult rat adipose tissue. Cell Tissue Res. 181:197–203. DOI: 10.1007/BF00219980. PMID: 195732.
Article31. Li CY, Wu XY, Tong JB, Yang XX, Zhao JL, Zheng QF, Zhao GB, Ma ZJ. 2015; Comparative analysis of human mesenchymal stem cells from bone marrow and adipose tissue under xeno-free conditions for cell therapy. Stem Cell Res Ther. 6:55. DOI: 10.1186/s13287-015-0066-5. PMID: 25884704. PMCID: PMC4453294.
Article32. Sakaguchi Y, Sekiya I, Yagishita K, Muneta T. 2005; Comparison of human stem cells derived from various mesenchymal tissues: superiority of synovium as a cell source. Arthritis Rheum. 52:2521–2529. DOI: 10.1002/art.21212. PMID: 16052568.
Article33. Fan J, Varshney RR, Ren L, Cai D, Wang DA. 2009; Synovium-derived mesenchymal stem cells: a new cell source for musculoskeletal regeneration. Tissue Eng Part B Rev. 15:75–86. DOI: 10.1089/ten.teb.2008.0586. PMID: 19196118.
Article34. To K, Zhang B, Romain K, Mak C, Khan W. 2019; Synovium-derived mesenchymal stem cell transplantation in cartilage regeneration: a PRISMA review of in vivo studies. Front Bioeng Biotechnol. 7:314. DOI: 10.3389/fbioe.2019.00314. PMID: 31803726. PMCID: PMC6873960.
Article35. Wang M, Yang Y, Yang D, Luo F, Liang W, Guo S, Xu J. 2009; The immunomodulatory activity of human umbilical cord blood-derived mesenchymal stem cells in vitro. Immu-nology. 126:220–232. DOI: 10.1111/j.1365-2567.2008.02891.x. PMID: 18624725. PMCID: PMC2632684.
Article36. Wobus AM, Boheler KR. 2005; Embryonic stem cells: prospects for developmental biology and cell therapy. Physiol Rev. 85:635–678. DOI: 10.1152/physrev.00054.2003. PMID: 15788707.
Article37. Mitsui K, Tokuzawa Y, Itoh H, Segawa K, Murakami M, Takahashi K, Maruyama M, Maeda M, Yamanaka S. 2003; The homeoprotein Nanog is required for maintenance of pluripotency in mouse epiblast and ES cells. Cell. 113:631–642. DOI: 10.1016/S0092-8674(03)00393-3. PMID: 12787504.
Article38. Takahashi K, Yamanaka S. 2006; Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 126:663–676. DOI: 10.1016/j.cell.2006.07.024. PMID: 16904174.
Article39. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI: 10.1016/j.cell.2007.11.019. PMID: 18035408.
Article40. Nakagawa M, Koyanagi M, Tanabe K, Takahashi K, Ichisaka T, Aoi T, Okita K, Mochiduki Y, Takizawa N, Yamanaka S. 2008; Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat Biotechnol. 26:101–106. DOI: 10.1038/nbt1374. PMID: 18059259.
Article41. Montserrat N, Ramírez-Bajo MJ, Xia Y, Sancho-Martinez I, Moya-Rull D, Miquel-Serra L, Yang S, Nivet E, Cortina C, González F, Izpisua Belmonte JC, Campistol JM. 2012; Generation of induced pluripotent stem cells from human renal proximal tubular cells with only two transcription factors, OCT4 and SOX2. J Biol Chem. 287:24131–24138. DOI: 10.1074/jbc.M112.350413. PMID: 22613719. PMCID: PMC3397840.
Article42. Lam MT, Longaker MT. 2012; Comparison of several attachment methods for human iPS, embryonic and adipose-derived stem cells for tissue engineering. J Tissue Eng Regen Med. 6 Suppl 3:s80–s86. DOI: 10.1002/term.1499. PMID: 22610948. PMCID: PMC4086291.
Article43. Volarevic V, Markovic BS, Gazdic M, Volarevic A, Jovicic N, Arsenijevic N, Armstrong L, Djonov V, Lako M, Stojkovic M. 2018; Ethical and safety issues of stem cell-based therapy. Int J Med Sci. 15:36–45. DOI: 10.7150/ijms.21666. PMID: 29333086. PMCID: PMC5765738.
Article44. Khan MA, Alanazi F, Ahmed HA, Shamma T, Kelly K, Hammad MA, Alawad AO, Assiri AM, Broering DC. 2019; iPSC-derived MSC therapy induces immune tolerance and supports long-term graft survival in mouse orthotopic tracheal transplants. Stem Cell Res Ther. 10:290. DOI: 10.1186/s13287-019-1397-4. PMID: 31547869. PMCID: PMC6757436.
Article45. Zhang J, Chan YC, Ho JC, Siu CW, Lian Q, Tse HF. 2012; Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via ether-à-go-go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol. 303:C115–C125. DOI: 10.1152/ajpcell.00326.2011. PMID: 22357737.
Article46. Meng X, Su RJ, Baylink DJ, Neises A, Kiroyan JB, Lee WY, Payne KJ, Gridley DS, Wang J, Lau KH, Li G, Zhang XB. 2013; Rapid and efficient reprogramming of human fetal and adult blood CD34+ cells into mesenchymal stem cells with a single factor. Cell Res. 23:658–672. DOI: 10.1038/cr.2013.40. PMID: 23478301. PMCID: PMC3641600.
Article47. Chen W, Baylink DJ, Lau KH, Zhang XB. 2016; Generation of mesenchymal stem cells by blood cell reprogramming. Curr Stem Cell Res Ther. 11:114–121. DOI: 10.2174/1574888X10666150531173448. PMID: 26027679.
Article48. Lee EJ, Kim M, Kim YD, Chung MJ, Elfadl A, Ulah HMA, Park D, Lee S, Park HS, Kim TH, Hwang D, Jeong KS. 2018; Establishment of stably expandable induced myogenic stem cells by four transcription factors. Cell Death Dis. 9:1092. DOI: 10.1038/s41419-018-1114-8. PMID: 30361642. PMCID: PMC6202407.
Article49. Steens J, Klein D. 2018; Current strategies to generate human mesenchymal stem cells in vitro. Stem Cells Int. 2018:6726185. DOI: 10.1155/2018/6726185. PMID: 30224922. PMCID: PMC6129345.
Article50. Huang CP, Chen CC, Shyr CR. 2018; Xenogeneic cell therapy provides a novel potential therapeutic option for cancers by restoring tissue function, repairing cancer wound and reviving anti-tumor immune responses. Cancer Cell Int. 18:9. DOI: 10.1186/s12935-018-0501-7. PMID: 29371832. PMCID: PMC5771064.
Article51. Liew A, O'Brien T. 2012; Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 3:28. DOI: 10.1186/scrt119. PMID: 22846185. PMCID: PMC3580466.
Article52. Obaid H, Connell D. 2010; Cell therapy in tendon disorders: what is the current evidence? Am J Sports Med. 38:2123–2132. DOI: 10.1177/0363546510373574. PMID: 20699425.53. Yin Z, Chen X, Chen JL, Ouyang HW. 2010; Stem cells for tendon tissue engineering and regeneration. Expert Opin Biol Ther. 10:689–700. DOI: 10.1517/14712591003769824. PMID: 20367125.
Article54. Fong EL, Chan CK, Goodman SB. 2011; Stem cell homing in musculoskeletal injury. Biomaterials. 32:395–409. DOI: 10.1016/j.biomaterials.2010.08.101. PMID: 20933277. PMCID: PMC2991369.
Article55. Diekman BO, Wu CL, Louer CR, Furman BD, Huebner JL, Kraus VB, Olson SA, Guilak F. 2013; Intra-articular delivery of purified mesenchymal stem cells from C57BL/6 or MRL/MpJ superhealer mice prevents posttraumatic arthritis. Cell Transplant. 22:1395–1408. DOI: 10.3727/096368912X653264. PMID: 22889498. PMCID: PMC3891895.
Article56. Huang TF, Yew TL, Chiang ER, Ma HL, Hsu CY, Hsu SH, Hsu YT, Hung SC. 2013; Mesenchymal stem cells from a hypoxic culture improve and engraft Achilles tendon repair. Am J Sports Med. 41:1117–1125. DOI: 10.1177/0363546513480786. PMID: 23539044.
Article57. Okamoto N, Kushida T, Oe K, Umeda M, Ikehara S, Iida H. 2010; Treating Achilles tendon rupture in rats with bone-marrow-cell transplantation therapy. J Bone Joint Surg Am. 92:2776–2284. DOI: 10.2106/JBJS.I.01325. PMID: 21123607.
Article58. Nourissat G, Diop A, Maurel N, Salvat C, Dumont S, Pigenet A, Gosset M, Houard X, Berenbaum F. 2010; Mesenchymal stem cell therapy regenerates the native bone-tendon junction after surgical repair in a degenerative rat model. PLoS One. 5:e12248. DOI: 10.1371/journal.pone.0012248. PMID: 20805884. PMCID: PMC2923611.
Article59. Suhaeb AM, Naveen S, Mansor A, Kamarul T. 2012; Hyaluronic acid with or without bone marrow derived-mesenchymal stem cells improves osteoarthritic knee changes in rat model: a preliminary report. Indian J Exp Biol. 50:383–390.60. Gupta PK, Chullikana A, Rengasamy M, Shetty N, Pandey V, Agarwal V, Wagh SY, Vellotare PK, Damodaran D, Viswanathan P, Thej C, Balasubramanian S, Majumdar AS. 2016; Efficacy and safety of adult human bone marrow-derived, cultured, pooled, allogeneic mesenchymal stromal cells (StempeucelⓇ): preclinical and clinical trial in osteoarthritis of the knee joint. Arthritis Res Ther. 18:301. DOI: 10.1186/s13075-016-1195-7. PMID: 27993154. PMCID: PMC5168586.61. Sato M, Uchida K, Nakajima H, Miyazaki T, Guerrero AR, Watanabe S, Roberts S, Baba H. 2012; Direct transplantation of mesenchymal stem cells into the knee joints of Hartley strain guinea pigs with spontaneous osteoarthritis. Arthritis Res Ther. 14:R31. DOI: 10.1186/ar3735. PMID: 22314040. PMCID: PMC3392826.
Article62. Kim SS, Kang MS, Lee KY, Lee MJ, Wang L, Kim HJ. 2012; Therapeutic effects of mesenchymal stem cells and hyaluronic acid injection on osteochondral defects in rabbits' knees. Knee Surg Relat Res. 24:164–172. DOI: 10.5792/ksrr.2012.24.3.164. PMID: 22977794. PMCID: PMC3438278.
Article63. Chiang ER, Ma HL, Wang JP, Liu CL, Chen TH, Hung SC. 2016; Allogeneic mesenchymal stem cells in combination with hyaluronic acid for the treatment of osteoarthritis in rabbits. PLoS One. 11:e0149835. DOI: 10.1371/journal.pone.0149835. PMID: 26915044. PMCID: PMC4767225.
Article64. Yang Q, Peng J, Lu SB, Guo QY, Zhao B, Zhang L, Wang AY, Xu WJ, Xia Q, Ma XL, Hu YC, Xu BS. 2011; Evaluation of an extracellular matrix-derived acellular biphasic scaffold/cell construct in the repair of a large articular high-load-bearing osteochondral defect in a canine model. Chin Med J (Engl). 124:3930–3938. PMID: 22340321.65. McIlwraith CW, Frisbie DD, Rodkey WG, Kisiday JD, Werpy NM, Kawcak CE, Steadman JR. 2011; Evaluation of intra-articular mesenchymal stem cells to augment healing of microfractured chondral defects. Arthroscopy. 27:1552–1561. DOI: 10.1016/j.arthro.2011.06.002. PMID: 21862278.
Article66. Al Faqeh H, Nor Hamdan BM, Chen HC, Aminuddin BS, Ruszymah BH. 2012; The potential of intra-articular injection of chondrogenic-induced bone marrow stem cells to retard the progression of osteoarthritis in a sheep model. Exp Gerontol. 47:458–464. DOI: 10.1016/j.exger.2012.03.018. PMID: 22759409.
Article67. Smith RK, Werling NJ, Dakin SG, Alam R, Goodship AE, Dudhia J. 2013; Beneficial effects of autologous bone marrow-derived mesenchymal stem cells in naturally occurring tendi-nopathy. PLoS One. 8:e75697. DOI: 10.1371/journal.pone.0075697. PMID: 24086616. PMCID: PMC3783421.
Article68. Godwin EE, Young NJ, Dudhia J, Beamish IC, Smith RK. 2012; Implantation of bone marrow-derived mesenchymal stem cells demonstrates improved outcome in horses with overstrain injury of the superficial digital flexor tendon. Equine Vet J. 44:25–32. DOI: 10.1111/j.2042-3306.2011.00363.x. PMID: 21615465.
Article69. Caniglia CJ, Schramme MC, Smith RK. 2012; The effect of intralesional injection of bone marrow derived mesenchymal stem cells and bone marrow supernatant on collagen fibril size in a surgical model of equine superficial digital flexor tendonitis. Equine Vet J. 44:587–593. DOI: 10.1111/j.2042-3306.2011.00514.x. PMID: 22150794.
Article70. Seo JP, Tanabe T, Tsuzuki N, Haneda S, Yamada K, Furuoka H, Tabata Y, Sasaki N. 2013; Effects of bilayer gelatin/β-tricalcium phosphate sponges loaded with mesenchymal stem cells, chondrocytes, bone morphogenetic protein-2, and platelet rich plasma on osteochondral defects of the talus in horses. Res Vet Sci. 95:1210–1216. DOI: 10.1016/j.rvsc.2013.08.016. PMID: 24054973.
Article71. Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Nuñez-Córdoba JM, Sánchez-Echenique C, Bondía JM, Aquerreta JD, Andreu EJ, Ornilla E, Villarón EM, Valentí-Azcárate A, Sánchez-Guijo F, Del Cañizo MC, Valentí-Nin JR, Prósper F. 2016; Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 14:246. DOI: 10.1186/s12967-016-0998-2. PMID: 27565858. PMCID: PMC5002157.
Article72. Lamo-Espinosa JM, Mora G, Blanco JF, Granero-Moltó F, Núñez-Córdoba JM, López-Elío S, Andreu E, Sánchez-Guijo F, Aquerreta JD, Bondía JM, Valentí-Azcárate A, Del Consuelo Del Cañizo M, Villarón EM, Valentí-Nin JR, Prósper F. 2018; Intra-articular injection of two different doses of autologous bone marrow mesenchymal stem cells versus hyaluronic acid in the treatment of knee osteoarthritis: long-term follow up of a multicenter randomized controlled clinical trial (phase I/II). J Transl Med. 16:213. DOI: 10.1186/s12967-018-1591-7. PMID: 30064455. PMCID: PMC6069715.
Article73. Vangsness CT Jr, Farr J 2nd, Boyd J, Dellaero DT, Mills CR, LeRoux-Williams M. 2014; Adult human mesenchymal stem cells delivered via intra-articular injection to the knee following partial medial meniscectomy: a randomized, double-blind, controlled study. J Bone Joint Surg Am. 96:90–98. DOI: 10.2106/JBJS.M.00058. PMID: 24430407.
Article74. Vega A, Martín-Ferrero MA, Del Canto F, Alberca M, García V, Munar A, Orozco L, Soler R, Fuertes JJ, Huguet M, Sánchez A, García-Sancho J. 2015; Treatment of knee osteoarthritis with allogeneic bone marrow mesenchymal stem cells: a randomized controlled trial. Transplantation. 99:1681–1690. DOI: 10.1097/TP.0000000000000678. PMID: 25822648.
Article75. Al-Najar M, Khalil H, Al-Ajlouni J, Al-Antary E, Hamdan M, Rahmeh R, Alhattab D, Samara O, Yasin M, Abdullah AA, Al-Jabbari E, Hmaid D, Jafar H, Awidi A. 2017; Intra-articular injection of expanded autologous bone marrow mesenchymal cells in moderate and severe knee osteoarthritis is safe: a phase I/II study. J Orthop Surg Res. 12:190. DOI: 10.1186/s13018-017-0689-6. PMID: 29233163. PMCID: PMC5727956.
Article76. Emadedin M, Aghdami N, Taghiyar L, Fazeli R, Moghadasali R, Jahangir S, Farjad R, Baghaban Eslaminejad M. 2012; Intra-articular injection of autologous mesenchymal stem cells in six patients with knee osteoarthritis. Arch Iran Med. 15:422–428. PMID: 22724879.77. Soler RR, Munar A, Soler RF, Peirau X, Huguet M, Alberca M, Sánchez A, García SJ, Orozco L. 2015; Treatment of knee osteoarthritis with autologous expanded bone marrow mesenchymal stem cells: 50 cases clinical and MRI results at one year follow-up. J Stem Cell Res Ther. 5:6. DOI: 10.4172/2157-7633.1000285.
Article78. Soler R, Orozco L, Munar A, Huguet M, López R, Vives J, Coll R, Codinach M, Garcia-Lopez J. 2016; Final results of a phase I-II trial using ex vivo expanded autologous Mesen-chymal Stromal Cells for the treatment of osteoarthritis of the knee confirming safety and suggesting cartilage regene-ration. Knee. 23:647–654. DOI: 10.1016/j.knee.2015.08.013. PMID: 26783191.
Article79. Mehrabani D, Mojtahed Jaberi F, Zakerinia M, Hadianfard MJ, Jalli R, Tanideh N, Zare S. 2016; The healing effect of bone marrow-derived stem cells in knee osteoarthritis: a case report. World J Plast Surg. 5:168–174. PMID: 27579273. PMCID: PMC5003953.80. Davatchi F, Abdollahi BS, Mohyeddin M, Shahram F, Nikbin B. 2011; Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int J Rheum Dis. 14:211–215. DOI: 10.1111/j.1756-185X.2011.01599.x. PMID: 21518322.
Article81. Orozco L, Munar A, Soler R, Alberca M, Soler F, Huguet M, Sentís J, Sánchez A, García-Sancho J. 2013; Treatment of knee osteoarthritis with autologous mesenchymal stem cells: a pilot study. Transplantation. 95:1535–1541. DOI: 10.1097/TP.0b013e318291a2da. PMID: 23680930.
Article82. Toghraie FS, Chenari N, Gholipour MA, Faghih Z, Torabinejad S, Dehghani S, Ghaderi A. 2011; Treatment of osteoarthritis with infrapatellar fat pad derived mesenchymal stem cells in rabbit. Knee. 18:71–75. DOI: 10.1016/j.knee.2010.03.001. PMID: 20591677.
Article83. Zaragoza MR, Serrato BC, Poveda JMC, Juncosa JJS, Bertomeu RC, Balletbo MG, Fariña MM, Guereño JMV. 2014; Force platform analysis of the effect of intrarticular injection of autologous adipose-derived mesenchymal stem cells associated to PRGF in osteoarthritic dogs. Osteoarthr Cartil. 22 Suppl:S198–S199. DOI: 10.1016/j.joca.2014.02.379.
Article84. Forcales SV. 2015; Potential of adipose-derived stem cells in muscular regenerative therapies. Front Aging Neurosci. 7:123. DOI: 10.3389/fnagi.2015.00123. PMID: 26217219. PMCID: PMC4499759.
Article85. Lee EM, Kim AY, Lee EJ, Park JK, Lee MM, Hwang M, Kim CY, Kim SY, Jeong KS. 2015; Therapeutic effects of mouse adipose-derived stem cells and losartan in the skeletal muscle of injured mdx mice. Cell Transplant. 24:939–953. DOI: 10.3727/096368914X678599. PMID: 24593934.
Article86. Song Y, Du H, Dai C, Zhang L, Li S, Hunter DJ, Lu L, Bao C. 2018; Human adipose-derived mesenchymal stem cells for osteoarthritis: a pilot study with long-term follow-up and repeated injections. Regen Med. 13:295–307. DOI: 10.2217/rme-2017-0152. PMID: 29417902.
Article87. Lu L, Dai C, Zhang Z, Du H, Li S, Ye P, Fu Q, Zhang L, Wu X, Dong Y, Song Y, Zhao D, Pang Y, Bao C. 2019; Treatment of knee osteoarthritis with intra-articular injection of autologous adipose-derived mesenchymal progenitor cells: a prospective, randomized, double-blind, active-controlled, phase IIb clinical trial. Stem Cell Res Ther. 10:143. DOI: 10.1186/s13287-019-1248-3. PMID: 31113476. PMCID: PMC6528322.
Article88. Zhao X, Ruan J, Tang H, Li J, Shi Y, Li M, Li S, Xu C, Lu Q, Dai C. 2019; Multi-compositional MRI evaluation of repair cartilage in knee osteoarthritis with treatment of allogeneic human adipose-derived mesenchymal progenitor cells. Stem Cell Res Ther. 10:308. DOI: 10.1186/s13287-019-1406-7. PMID: 31639063. PMCID: PMC6805685.
Article89. Lee JC, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. 2013; Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 29:1034–1046. DOI: 10.1016/j.arthro.2013.02.026. PMID: 23726109.
Article90. Shimomura K, Moriguchi Y, Ando W, Nansai R, Fujie H, Hart DA, Gobbi A, Kita K, Horibe S, Shino K, Yoshikawa H, Nakamura N. 2014; Osteochondral repair using a scaffold-free tissue-engineered construct derived from synovial mesenchymal stem cells and a hydroxyapatite-based artificial bone. Tissue Eng Part A. 20:2291–2304. DOI: 10.1089/ten.tea.2013.0414. PMID: 24655056.
Article91. Shimomura K, Ando W, Tateishi K, Nansai R, Fujie H, Hart DA, Kohda H, Kita K, Kanamoto T, Mae T, Nakata K, Shino K, Yoshikawa H, Nakamura N. 2010; The influence of skeletal maturity on allogenic synovial mesenchymal stem cell-based repair of cartilage in a large animal model. Biomaterials. 31:8004–8011. DOI: 10.1016/j.biomaterials.2010.07.017. PMID: 20674010.
Article92. Nakamura T, Sekiya I, Muneta T, Kobayashi E. 2013; Articular cartilage regenerative therapy with synovial mesenchymal stem cells in a pig model. Clin Calcium. 23:1741–1749. Japanese. PMID: 24292528.93. Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, Librach F, Buerchler S, Friedman MS, Walker NJ, Borjesson DL. 2011; Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy. 13:419–430. DOI: 10.3109/14653249.2010.536213. PMID: 21105841.
Article94. Van Loon VJ, Scheffer CJ, Genn HJ, Hoogendoorn AC, Greve JW. 2014; Clinical follow-up of horses treated with allogeneic equine mesenchymal stem cells derived from umbilical cord blood for different tendon and ligament disorders. Vet Q. 34:92–97. DOI: 10.1080/01652176.2014.949390. PMID: 25072527.
Article95. Jeong SY, Kim DH, Ha J, Jin HJ, Kwon SJ, Chang JW, Choi SJ, Oh W, Yang YS, Kim G, Kim JS, Yoon JR, Cho DH, Jeon HB. 2013; Thrombospondin-2 secreted by human umbilical cord blood-derived mesenchymal stem cells promotes chondrogenic differentiation. Stem Cells. 31:2136–2148. DOI: 10.1002/stem.1471. PMID: 23843355.
Article96. Kwon DR, Park GY, Lee SC. 2019; Regenerative effects of mesenchymal stem cells by dosage in a chronic rotator cuff tendon tear in a rabbit model. Regen Med. 14:1001–1012. DOI: 10.2217/rme-2018-0125. PMID: 31726959.
Article97. An JH, Park H, Song JA, Ki KH, Yang JY, Choi HJ, Cho SW, Kim SW, Kim SY, Yoo JJ, Baek WY, Kim JE, Choi SJ, Oh W, Shin CS. 2013; Transplantation of human umbilical cord blood-derived mesenchymal stem cells or their conditioned medium prevents bone loss in ovariectomized nude mice. Tissue Eng Part A. 19:685–696. DOI: 10.1089/ten.tea.2012.0047. PMID: 23215868. PMCID: PMC3568969.
Article98. Song JS, Hong KT, Kim NM, Jung JY, Park HS, Lee SH, Cho YJ, Kim SJ. 2020; Implantation of allogenic umbilical cord blood-derived mesenchymal stem cells improves knee osteoarthritis outcomes: two-year follow-up. Regen Ther. 14:32–39. DOI: 10.1016/j.reth.2019.10.003. PMID: 31988992. PMCID: PMC6965506.
Article99. Jung Y, Bauer G, Nolta JA. 2012; Concise review: induced pluripotent stem cell-derived mesenchymal stem cells: progress toward safe clinical products. Stem Cells. 30:42–47. DOI: 10.1002/stem.727. PMID: 21898694. PMCID: PMC3784250.
Article100. Jungbluth P, Spitzhorn LS, Grassmann J, Tanner S, Latz D, Rahman MS, Bohndorf M, Wruck W, Sager M, Grotheer V, Kröpil P, Hakimi M, Windolf J, Schneppendahl J, Adjaye J. 2019; Human iPSC-derived iMSCs improve bone regeneration in mini-pigs. Bone Res. 7:32. DOI: 10.1038/s41413-019-0069-4. PMID: 31667001. PMCID: PMC6813363.
Article101. Lin L, Bolund L, Luo Y. 2016; Towards personalized regene-rative cell therapy: mesenchymal stem cells derived from human induced pluripotent stem cells. Curr Stem Cell Res Ther. 11:122–130. DOI: 10.2174/1574888X10666150723150236. PMID: 26201862.
Article102. Kim IG, Park SA, Lee SH, Choi JS, Cho H, Lee SJ, Kwon YW, Kwon SK. 2020; Transplantation of a 3D-printed tracheal graft combined with iPS cell-derived MSCs and chondro-cytes. Sci Rep. 10:4326. DOI: 10.1038/s41598-020-61405-4. PMID: 32152475. PMCID: PMC7062776.
Article103. Chacón-Martínez CA, Koester J, Wickström SA. 2018; Signaling in the stem cell niche: regulating cell fate, function and plasticity. Development. 145:dev165399. DOI: 10.1242/dev.165399. PMID: 30068689.
Article104. Park JK, Ki MR, Lee EM, Kim AY, You SY, Han SY, Lee EJ, Hong IH, Kwon SH, Kim SJ, Rando TA, Jeong KS. 2012; Losartan improves adipose tissue-derived stem cell niche by inhibiting transforming growth factor-β and fibrosis in skeletal muscle injury. Cell Transplant. 21:2407–2424. DOI: 10.3727/096368912X637055. PMID: 22507443.
Article105. Shcherbata HR. 2019; miRNA functions in stem cells and their niches: lessons from the Drosophila ovary. Curr Opin Insect Sci. 31:29–36. DOI: 10.1016/j.cois.2018.07.006. PMID: 31109670.
Article106. Sims PJ, Faioni EM, Wiedmer T, Shattil SJ. 1988; Complement proteins C5b-9 cause release of membrane vesicles from the platelet surface that are enriched in the membrane receptor for coagulation factor Va and express prothrombinase activity. J Biol Chem. 263:18205–18212. DOI: 10.1016/S0021-9258(19)81346-7. PMID: 2848029.
Article107. Satta N, Toti F, Feugeas O, Bohbot A, Dachary-Prigent J, Eschwège V, Hedman H, Freyssinet JM. 1994; Monocyte vesiculation is a possible mechanism for dissemination of membrane-associated procoagulant activities and adhesion molecules after stimulation by lipopolysaccharide. J Immunol. 153:3245–3255. PMID: 7522256.108. Tricarico C, Clancy J, D'Souza-Schorey C. 2017; Biology and biogenesis of shed microvesicles. Small GTPases. 8:220–232. DOI: 10.1080/21541248.2016.1215283. PMID: 27494381. PMCID: PMC5680703.
Article109. Pan BT, Teng K, Wu C, Adam M, Johnstone RM. 1985; Electron microscopic evidence for externalization of the transferrin receptor in vesicular form in sheep reticulocytes. J Cell Biol. 101:942–948. DOI: 10.1083/jcb.101.3.942. PMID: 2993317. PMCID: PMC2113705.
Article110. Meirelles Lda S, Fontes AM, Covas DT, Caplan AI. 2009; Mechanisms involved in the therapeutic properties of mesenchymal stem cells. Cytokine Growth Factor Rev. 20:419–427. DOI: 10.1016/j.cytogfr.2009.10.002. PMID: 19926330.
Article111. Deng H, Sun C, Sun Y, Li H, Yang L, Wu D, Gao Q, Jiang X. 2018; Lipid, protein, and microRNA composition within mesenchymal stem cell-derived exosomes. Cell Reprogram. 20:178–186. DOI: 10.1089/cell.2017.0047. PMID: 29782191.
Article112. Choi DS, Kim DK, Kim YK, Gho YS. 2013; Proteomics, transcriptomics and lipidomics of exosomes and ectosomes. Proteomics. 13:1554–1571. DOI: 10.1002/pmic.201200329. PMID: 23401200.
Article113. Choi DS, Kim DK, Kim YK, Gho YS. 2015; Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom Rev. 34:474–490. DOI: 10.1002/mas.21420. PMID: 24421117.
Article114. Mathivanan S, Simpson RJ. 2009; ExoCarta: a compendium of exosomal proteins and RNA. Proteomics. 9:4997–5000. DOI: 10.1002/pmic.200900351. PMID: 19810033.
Article115. Ling H, Fabbri M, Calin GA. 2013; MicroRNAs and other non-coding RNAs as targets for anticancer drug development. Nat Rev Drug Discov. 12:847–865. DOI: 10.1038/nrd4140. PMID: 24172333. PMCID: PMC4548803.
Article116. Xin H, Li Y, Chopp M. 2014; Exosomes/miRNAs as mediating cell-based therapy of stroke. Front Cell Neurosci. 8:377. DOI: 10.3389/fncel.2014.00377. PMID: 25426026. PMCID: PMC4226157.
Article117. EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. 2013; Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 12:347–357. DOI: 10.1038/nrd3978. PMID: 23584393.
Article118. Feng Y, Huang W, Wani M, Yu X, Ashraf M. 2014; Ischemic preconditioning potentiates the protective effect of stem cells through secretion of exosomes by targeting Mecp2 via miR-22. PLoS One. 9:e88685. DOI: 10.1371/journal.pone.0088685. PMID: 24558412. PMCID: PMC3928277.
Article119. Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, Miyado K, Higashi Y, Ochi M. 2016; Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med. 5:1620–1630. DOI: 10.5966/sctm.2015-0285. PMID: 27460850. PMCID: PMC5189643.
Article120. Nakamura Y, Miyaki S, Ishitobi H, Matsuyama S, Nakasa T, Kamei N, Akimoto T, Higashi Y, Ochi M. 2015; Mesenchymal-stem-cell-derived exosomes accelerate skeletal muscle rege-neration. FEBS Lett. 589:1257–1265. DOI: 10.1016/j.febslet.2015.03.031. PMID: 25862500.
Article121. Liu X, Yang Y, Li Y, Niu X, Zhao B, Wang Y, Bao C, Xie Z, Lin Q, Zhu L. 2017; Integration of stem cell-derived exosomes with in situ hydrogel glue as a promising tissue patch for articular cartilage regeneration. Nanoscale. 9:4430–4438. DOI: 10.1039/C7NR00352H. PMID: 28300264.
Article122. Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, Hu B, Wang Y, Li X. 2016; Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 12:836–849. DOI: 10.7150/ijbs.14809. PMID: 27313497. PMCID: PMC4910602.
Article123. Hu GW, Li Q, Niu X, Hu B, Liu J, Zhou SM, Guo SC, Lang HL, Zhang CQ, Wang Y, Deng ZF. 2015; Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells attenuate limb ischemia by promoting angiogenesis in mice. Stem Cell Res Ther. 6:10. DOI: 10.1186/scrt546. PMID: 26268554. PMCID: PMC4533800.
Article124. Liu X, Li Q, Niu X, Hu B, Chen S, Song W, Ding J, Zhang C, Wang Y. 2017; Exosomes secreted from human-induced pluripotent stem cell-derived mesenchymal stem cells prevent osteonecrosis of the femoral head by promoting angio-genesis. Int J Biol Sci. 13:232–244. DOI: 10.7150/ijbs.16951. PMID: 28255275. PMCID: PMC5332877.
Article125. Zhu Y, Wang Y, Zhao B, Niu X, Hu B, Li Q, Zhang J, Ding J, Chen Y, Wang Y. 2017; Comparison of exosomes secreted by induced pluripotent stem cell-derived mesenchymal stem cells and synovial membrane-derived mesenchymal stem cells for the treatment of osteoarthritis. Stem Cell Res Ther. 8:64. DOI: 10.1186/s13287-017-0510-9. PMID: 28279188. PMCID: PMC5345222.
Article126. Sabapathy V, Kumar S. 2016; hiPSC-derived iMSCs: NextGen MSCs as an advanced therapeutically active cell resource for regenerative medicine. J Cell Mol Med. 20:1571–1588. DOI: 10.1111/jcmm.12839. PMID: 27097531. PMCID: PMC4956943.
Article127. Zhang Y, Liang X, Liao S, Wang W, Wang J, Li X, Ding Y, Liang Y, Gao F, Yang M, Fu Q, Xu A, Chai YH, He J, Tse HF, Lian Q. 2015; Potent paracrine effects of human induced pluripotent stem cell-derived mesenchymal stem cells attenuate doxorubicin-induced cardiomyopathy. Sci Rep. 5:11235. DOI: 10.1038/srep11235. PMID: 26057572. PMCID: PMC4460911.
Article128. Katsuda T, Tsuchiya R, Kosaka N, Yoshioka Y, Takagaki K, Oki K, Takeshita F, Sakai Y, Kuroda M, Ochiya T. 2013; Human adipose tissue-derived mesenchymal stem cells secrete functional neprilysin-bound exosomes. Sci Rep. 3:1197. DOI: 10.1038/srep01197. PMID: 23378928. PMCID: PMC3561625.
Article129. Del Fattore A, Luciano R, Saracino R, Battafarano G, Rizzo C, Pascucci L, Alessandri G, Pessina A, Perrotta A, Fierabracci A, Muraca M. 2015; Differential effects of extracellular vesicles secreted by mesenchymal stem cells from different sources on glioblastoma cells. Expert Opin Biol Ther. 15:495–504. DOI: 10.1517/14712598.2015.997706. PMID: 25539575.
Article130. Lopez-Verrilli MA, Caviedes A, Cabrera A, Sandoval S, Wyneken U, Khoury M. 2016; Mesenchymal stem cell-derived exosomes from different sources selectively promote neuritic outgrowth. Neuroscience. 320:129–139. DOI: 10.1016/j.neuroscience.2016.01.061. PMID: 26851773.
Article131. Christopher AF, Kaur RP, Kaur G, Kaur A, Gupta V, Bansal P. 2016; MicroRNA therapeutics: discovering novel targets and developing specific therapy. Perspect Clin Res. 7:68–74. DOI: 10.4103/2229-3485.179431. PMID: 27141472. PMCID: PMC4840794.
Article132. Johnnidis JB, Harris MH, Wheeler RT, Stehling-Sun S, Lam MH, Kirak O, Brummelkamp TR, Fleming MD, Camargo FD. 2008; Regulation of progenitor cell proliferation and granulocyte function by microRNA-223. Nature. 451:1125–1129. DOI: 10.1038/nature06607. PMID: 18278031.
Article133. Sun L, Wu Z, Shao Y, Pu Y, Miu W, Yao J, Wu Y, Yang Z. 2012; MicroRNA-34a suppresses cell proliferation and induces apoptosis in U87 glioma stem cells. Technol Cancer Res Treat. 11:483–490. DOI: 10.7785/tcrt.2012.500264. PMID: 22568628.
Article134. Anderson RM. 2012; A role for dicer in aging and stress survival. Cell Metab. 16:285–286. DOI: 10.1016/j.cmet.2012.08.006. PMID: 22958915. PMCID: PMC3465679.
Article135. Höbel S, Aigner A. 2013; Polyethylenimines for siRNA and miRNA delivery in vivo. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 5:484–501. DOI: 10.1002/wnan.1228. PMID: 23720168.136. Fasanaro P, Greco S, Ivan M, Capogrossi MC, Martelli F. 2010; microRNA: emerging therapeutic targets in acute ischemic diseases. Pharmacol Ther. 125:92–104. DOI: 10.1016/j.pharmthera.2009.10.003. PMID: 19896977.
Article137. Pereira DM, Rodrigues PM, Borralho PM, Rodrigues CM. 2013; Delivering the promise of miRNA cancer therapeutics. Drug Discov Today. 18:282–289. DOI: 10.1016/j.drudis.2012.10.002. PMID: 23064097.
Article138. Lv H, Zhang S, Wang B, Cui S, Yan J. 2006; Toxicity of cationic lipids and cationic polymers in gene delivery. J Control Release. 114:100–109. DOI: 10.1016/j.jconrel.2006.04.014. PMID: 16831482.
Article139. Zhang Y, Wang Z, Gemeinhart RA. 2013; Progress in microRNA delivery. J Control Release. 172:962–974. DOI: 10.1016/j.jconrel.2013.09.015. PMID: 24075926. PMCID: PMC3891846.
Article140. Lee SY, Huh MS, Lee S, Lee SJ, Chung H, Park JH, Oh YK, Choi K, Kim K, Kwon IC. 2010; Stability and cellular uptake of polymerized siRNA (poly-siRNA)/polyethylenimine (PEI) complexes for efficient gene silencing. J Control Release. 141:339–346. DOI: 10.1016/j.jconrel.2009.10.007. PMID: 19836427.
Article141. Lee SWL, Paoletti C, Campisi M, Osaki T, Adriani G, Kamm RD, Mattu C, Chiono V. 2019; MicroRNA delivery through nanoparticles. J Control Release. 313:80–95. DOI: 10.1016/j.jconrel.2019.10.007. PMID: 31622695. PMCID: PMC6900258.
Article142. Álvarez-Viejo M. 2020; Mesenchymal stem cells from different sources and their derived exosomes: a pre-clinical per-spective. World J Stem Cells. 12:100–109. DOI: 10.4252/wjsc.v12.i2.100. PMID: 32184935. PMCID: PMC7062037.
Article143. Kaur S, Abu-Shahba AG, Paananen RO, Hongisto H, Hiidenmaa H, Skottman H, Seppänen-Kaijansinkko R, Mannerström B. 2018; Small non-coding RNA landscape of extracellular vesicles from human stem cells. Sci Rep. 8:15503. DOI: 10.1038/s41598-018-33899-6. PMID: 30341351. PMCID: PMC6195565.
Article144. Hanna J, Hossain GS, Kocerha J. 2019; The potential for microRNA therapeutics and clinical research. Front Genet. 10:478. DOI: 10.3389/fgene.2019.00478. PMID: 31156715. PMCID: PMC6532434.
Article145. Cosenza S, Ruiz M, Toupet K, Jorgensen C, Noël D. 2017; Mesenchymal stem cells derived exosomes and microparticles protect cartilage and bone from degradation in osteoarthritis. Sci Rep. 7:16214. DOI: 10.1038/s41598-017-15376-8. PMID: 29176667. PMCID: PMC5701135.
Article146. Xu JF, Yang GH, Pan XH, Zhang SJ, Zhao C, Qiu BS, Gu HF, Hong JF, Cao L, Chen Y, Xia B, Bi Q, Wang YP. 2014; Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 9:e114627. DOI: 10.1371/journal.pone.0114627. PMID: 25503309. PMCID: PMC4263734.
Article147. Mao G, Zhang Z, Hu S, Zhang Z, Chang Z, Huang Z, Liao W, Kang Y. 2018; Exosomes derived from miR-92a-3p-overexpressing human mesenchymal stem cells enhance chondrogenesis and suppress cartilage degradation via targeting WNT5A. Stem Cell Res Ther. 9:247. DOI: 10.1186/s13287-018-1004-0. PMID: 30257711. PMCID: PMC6158854.
Article148. Wang R, Xu B, Xu H. 2018; TGF-β1 promoted chondrocyte proliferation by regulating Sp1 through MSC-exosomes derived miR-135b. Cell Cycle. 17:2756–2765. DOI: 10.1080/15384101.2018.1556063. PMID: 30526325. PMCID: PMC6343719.
Article149. Sun H, Hu S, Zhang Z, Lun J, Liao W, Zhang Z. 2019; Expression of exosomal microRNAs during chondrogenic differentiation of human bone mesenchymal stem cells. J Cell Biochem. 120:171–181. DOI: 10.1002/jcb.27289. PMID: 30277597.
Article150. Zhang X, Chang A, Li Y, Gao Y, Wang H, Ma Z, Li X, Wang B. 2015; miR-140-5p regulates adipocyte differentiation by targeting transforming growth factor-β signaling. Sci Rep. 5:18118. DOI: 10.1038/srep18118. PMID: 26657345. PMCID: PMC4676041.
Article151. Wei J, Li H, Wang S, Li T, Fan J, Liang X, Li J, Han Q, Zhu L, Fan L, Zhao RC. 2014; let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 23:1452–1463. DOI: 10.1089/scd.2013.0600. PMID: 24617339. PMCID: PMC4066225.
Article152. Zhang WB, Zhong WJ, Wang L. 2014; A signal-amplification circuit between miR-218 and Wnt/β-catenin signal promotes human adipose tissue-derived stem cells osteogenic diffe-rentiation. Bone. 58:59–66. DOI: 10.1016/j.bone.2013.09.015. PMID: 24091133.
Article153. Wu J, Kuang L, Chen C, Yang J, Zeng WN, Li T, Chen H, Huang S, Fu Z, Li J, Liu R, Ni Z, Chen L, Yang L. 2019; miR-100-5p-abundant exosomes derived from infrapatellar fat pad MSCs protect articular cartilage and ameliorate gait abnormalities via inhibition of mTOR in osteoarthritis. Biomaterials. 206:87–100. DOI: 10.1016/j.biomaterials.2019.03.022. PMID: 30927715.
Article154. Bonafede R, Scambi I, Peroni D, Potrich V, Boschi F, Benati D, Bonetti B, Mariotti R. 2016; Exosome derived from murine adipose-derived stromal cells: neuroprotective effect on in vitro model of amyotrophic lateral sclerosis. Exp Cell Res. 340:150–158. DOI: 10.1016/j.yexcr.2015.12.009. PMID: 26708289.
Article155. Li W, Liu Y, Zhang P, Tang Y, Zhou M, Jiang W, Zhang X, Wu G, Zhou Y. 2018; Tissue-engineered bone immobilized with human adipose stem cells-derived exosomes promotes bone regeneration. ACS Appl Mater Interfaces. 10:5240–5254. DOI: 10.1021/acsami.7b17620. PMID: 29359912.
Article156. Tao SC, Yuan T, Zhang YL, Yin WJ, Guo SC, Zhang CQ. 2017; Exosomes derived from miR-140-5p-overexpressing human synovial mesenchymal stem cells enhance cartilage tissue regeneration and prevent osteoarthritis of the knee in a rat model. Theranostics. 7:180–195. DOI: 10.7150/thno.17133. PMID: 28042326. PMCID: PMC5196895.
Article157. Wen S, Dooner M, Cheng Y, Papa E, Del Tatto M, Pereira M, Deng Y, Goldberg L, Aliotta J, Chatterjee D, Stewart C, Carpanetto A, Collino F, Bruno S, Camussi G, Quesenberry P. 2016; Mesenchymal stromal cell-derived extracellular vesicles rescue radiation damage to murine marrow hematopoietic cells. Leukemia. 30:2221–2231. DOI: 10.1038/leu.2016.107. PMID: 27150009. PMCID: PMC5093052.
Article158. Ribeiro A, Laranjeira P, Mendes S, Velada I, Leite C, Andrade P, Santos F, Henriques A, Grãos M, Cardoso CM, Martinho A, Pais M, da Silva CL, Cabral J, Trindade H, Paiva A. 2013; Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 4:125. DOI: 10.1186/scrt336. PMID: 24406104. PMCID: PMC3854702.
Article159. Montesinos JJ, Flores-Figueroa E, Castillo-Medina S, Flores-Guzmán P, Hernández-Estévez E, Fajardo-Orduña G, Orozco S, Mayani H. 2009; Human mesenchymal stromal cells from adult and neonatal sources: comparative analysis of their morphology, immunophenotype, differentiation patterns and neural protein expression. Cytotherapy. 11:163–176. DOI: 10.1080/14653240802582075. PMID: 19152152.
Article160. Prasanna SJ, Gopalakrishnan D, Shankar SR, Vasandan AB. 2010; Pro-inflammatory cytokines, IFNgamma and TNFalpha, influence immune properties of human bone marrow and Wharton jelly mesenchymal stem cells differentially. PLoS One. 5:e9016. DOI: 10.1371/journal.pone.0009016. PMID: 20126406. PMCID: PMC2814860.161. Wang X, Kimbrel EA, Ijichi K, Paul D, Lazorchak AS, Chu J, Kouris NA, Yavanian GJ, Lu SJ, Pachter JS, Crocker SJ, Lanza R, Xu RH. 2014; Human ESC-derived MSCs outperform bone marrow MSCs in the treatment of an EAE model of multiple sclerosis. Stem Cell Reports. 3:115–130. DOI: 10.1016/j.stemcr.2014.04.020. PMID: 25068126. PMCID: PMC4110787.
Article162. Wegmeyer H, Bröske AM, Leddin M, Kuentzer K, Nisslbeck AK, Hupfeld J, Wiechmann K, Kuhlen J, von Schwerin C, Stein C, Knothe S, Funk J, Huss R, Neubauer M. 2013; Mesenchymal stromal cell characteristics vary depending on their origin. Stem Cells Dev. 22:2606–2618. DOI: 10.1089/scd.2013.0016. PMID: 23676112. PMCID: PMC3780294.
Article163. Zhang ZY, Teoh SH, Chong MS, Schantz JT, Fisk NM, Choolani MA, Chan J. 2009; Superior osteogenic capacity for bone tissue engineering of fetal compared with perinatal and adult mesenchymal stem cells. Stem Cells. 27:126–137. DOI: 10.1634/stemcells.2008-0456. PMID: 18832592.
Article164. Chang MG, Tung L, Sekar RB, Chang CY, Cysyk J, Dong P, Marbán E, Abraham MR. 2006; Proarrhythmic potential of mesenchymal stem cell transplantation revealed in an in vitro coculture model. Circulation. 113:1832–1841. DOI: 10.1161/CIRCULATIONAHA.105.593038. PMID: 16606790.
Article165. Breitbach M, Bostani T, Roell W, Xia Y, Dewald O, Nygren JM, Fries JW, Tiemann K, Bohlen H, Hescheler J, Welz A, Bloch W, Jacobsen SE, Fleischmann BK. 2007; Potential risks of bone marrow cell transplantation into infarcted hearts. Blood. 110:1362–1369. DOI: 10.1182/blood-2006-12-063412. PMID: 17483296.
Article166. Yao X, Wei W, Wang X, Chenglin L, Björklund M, Ouyang H. 2019; Stem cell derived exosomes: microRNA therapy for age-related musculoskeletal disorders. Biomaterials. 224:119492. DOI: 10.1016/j.biomaterials.2019.119492. PMID: 31557588.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The Role of microRNAs Involved in Mesenchymal Stem Cell Differentiation
- Current Trends and Prospect of Cell Therapy using Hematopoietic Stem Cells
- Stem cells in musculoskeletal system for clinical application
- Recent Trends and Strategies in Stem Cell Therapy for Alzheimer's Disease
- Clinical utilization of cord blood over human health: experience of stem cell transplantation and cell therapy using cord blood in Korea