Anesth Pain Med.
2021 Apr;16(2):177-183. 10.17085/apm.20093.
Real world adverse events of interspinous spacers using Manufacturer and User Facility Device Experience data
- Affiliations
-
- 1Department of Anesthesiology, Yale New Haven Hospital, New Haven, CT, USA
- KMID: 2515544
- DOI: http://doi.org/10.17085/apm.20093
Abstract
- Background
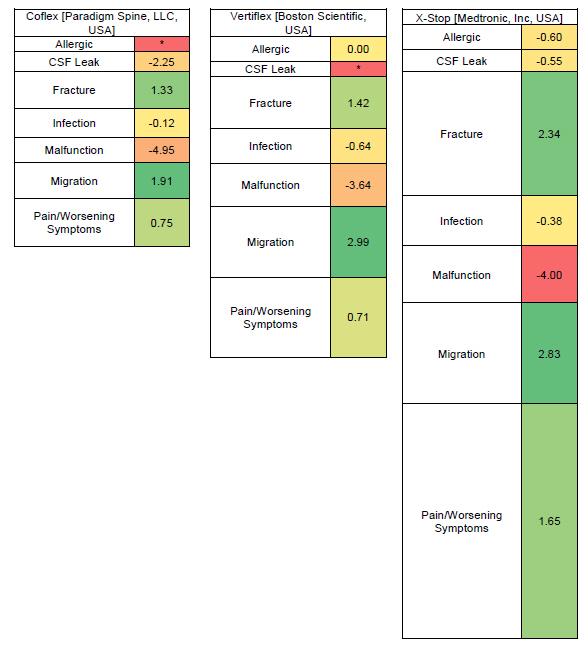
Lumbar spinal stenosis is a condition of progressive neurogenic claudication that can be managed with lumbar decompression surgery or less invasive interspinous process devices after failed conservative therapy. Popular interspinous process spacers include X-Stop, Vertiflex and Coflex, with X-Stop being taken off market due to its adverse events profile.
Methods
A disproportionality analysis was conducted to determine whether a statistically significant signal exists in the three interspinous spacers and the reported adverse events using the Manufacturer and User Facility Device Experience (MAUDE) database maintained by the US Food and Drug Administration.
Results
Statistically significant signals were found with each of the three interspinous spacer devices (Coflex, Vertiflex, and X-Stop) and each of the following adverse events: fracture, migration, and pain/worsening symptoms.
Conclusions
Further studies such as randomized controlled trials are needed to validate the findings.
Keyword
Figure
Reference
-
1. Proietti L, Scaramuzzo L, Schiro' GR, Sessa S, Logroscino CA. Complications in lumbar spine surgery: a retrospective analysis. Indian J Orthop. 2013; 47:340–5.2. Hartman J, Granville M, Jacobson RE. The use of Vertiflex® interspinous spacer device in patients with lumbar spinal stenosis and concurrent medical comorbidities. Cureus. 2019; 11:e5374.3. Manufacturer and User Facility Device Experience. U.S. Food and Drug Administration [serial on the Internet]. [cited 2020 Jun 1]. Available from https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfMAUDE/Search.cfm?smc=1.4. Tamura T, Sakaeda T, Kadoyama K, Okuno Y. Omeprazole- and esomeprazole-associated hypomagnesaemia: data mining of the public version of the FDA Adverse Event Reporting System. Int J Med Sci. 2012; 9:322–6.5. van Puijenbroek EP, van Grootheest K, Diemont WL, Leufkens HG, Egberts AC. Determinants of signal selection in a spontaneous reporting system for adverse drug reactions. Br J Clin Pharmacol. 2001; 52:579–86.6. Evans SJ, Waller PC, Davis S. Use of proportional reporting ratios (PRRs) for signal generation from spontaneous adverse drug reaction reports. Pharmacoepidemiol Drug Saf. 2001; 10:483–6.7. Bate A, Lindquist M, Edwards IR, Olsson S, Orre R, Lansner A, et al. A Bayesian neural network method for adverse drug reaction signal generation. Eur J Clin Pharmacol. 1998; 54:315–21.8. Belamalem S, Khadmaoui A, Tailibi I, Benkirane R, Soulaymani BR, Soulaymani A. Use of measures of disproportionality in pharmacovigilance. Biotechnol (Rajkot). 2016; 12:193–7.9. Vivekanandan K, Tripathi A, Saurabh A, Kumar R, Kumar R, Prasad T, et al. Quantitative methods for the identification of signals for individual case safety reports in India. Ther Innov Regul Sci. 2015; 49:898–902.10. Böhm R. Primer on disproportionality analysis. OpenVigil [serial on the Internet]. 2018 Oct 16 [cited 2020 Jun 1]. Available from http://openvigil.sourceforge.net/doc/DPA.pdf.11. Mo Z, Li D, Zhang R, Chang M, Yang B, Tang S. Comparative effectiveness and safety of posterior lumbar interbody fusion, Coflex, Wallis, and X-stop for lumbar degenerative diseases: a systematic review and network meta-analysis. Clin Neurol Neurosurg. 2018; 172:74–81.12. Deer TR, Grider JS, Pope JE, Falowski S, Lamer TJ, Calodney A, et al. The MIST Guidelines: the Lumbar Spinal Stenosis Consensus Group guidelines for minimally invasive spine treatment. Pain Pract. 2019; 19:250–74.13. Bini W, Miller LE, Block JE. Minimally invasive treatment of moderate lumbar spinal stenosis with the superion interspinous spacer. Open Orthop J. 2011; 5:361–7.14. Patel VV, Whang PG, Haley TR, Bradley WD, Nunley PD, Davis RP, et al. Superion interspinous process spacer for intermittent neurogenic claudication secondary to moderate lumbar spinal stenosis: two-year results from a randomized controlled FDA-IDE pivotal trial. Spine (Phila Pa 1976). 2015; 40:275–82.15. Bowers C, Amini A, Dailey AT, Schmidt MH. Dynamic interspinous process stabilization: review of complications associated with the X-Stop device. Neurosurg Focus. 2010; 28:E8.16. Ma X, Ma T, Dang X, Shi J, Niu N, Chang L, et al. Comparison of two interspinous spacers for treatment of moderate lumbar spinal stenosis: a meta-analysis of prospective randomized controlled trials. Int J Clin Exp Med. 2017; 10:4497–507.17. Aggarwal P. Pyoderma gangrenosum adverse event with Rituximab use: a postmarketing pharmacovigilance analysis. Dermatol Ther. 2020; 33:e13221.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Adverse Events Associated With Synthetic Male Slings: An Analysis of the Food and Drug Administration Manufacturer and User Facility Device Experience Database
- An evaluation of the Manufacturer And User Facility Device Experience database that inspired the United States Food and Drug Administration's Reclassification of transvaginal mesh
- Adverse Events in Total Artificial Heart for End-Stage Heart Failure: Insight From the Food and Drug Administration Manufacturer and User Facility Device Experience (MAUDE)
- Analysis of reported adverse events of pipeline stents for intracranial aneurysms using the FDA MAUDE database
- A Morphometric Study of the Lumbar Interspinous Space in 100 Stanford University Medical Center Patients