Anesth Pain Med.
2021 Apr;16(2):151-157. 10.17085/apm.20069.
Preoperative cephalhematoma size measured with computed tomography predicts intraoperative bleeding in pediatric patients undergoing cranioplasty
- Affiliations
-
- 1Department of Anesthesiology and Pain Medicine, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- 2Anesthesia and Pain Research Institute, Yonsei University College of Medicine, Seoul, Korea
- 3Department of Pediatric Neurosurgery, Severance Children’s Hospital, Yonsei University College of Medicine, Seoul, Korea
- 4Department of Radiology, Severance Hospital, Yonsei University College of Medicine, Seoul, Korea
- KMID: 2515540
- DOI: http://doi.org/10.17085/apm.20069
Abstract
- Background
Cranioplasty for the treatment of cephalhematomas in small infants with limited blood volume is challenging because of massive bleeding. This study aimed to elucidate the correlation between cephalhematoma size and intraoperative blood loss and identify criteria that can predict large intraoperative blood loss.
Methods
We reviewed the medical records of 120 pediatric patients aged less than 24 months who underwent cranioplasty for treatment of a cephalhematoma. The cephalhematoma sizes in preoperative brain computed tomography (CT) were measured using ImageJ.
Results
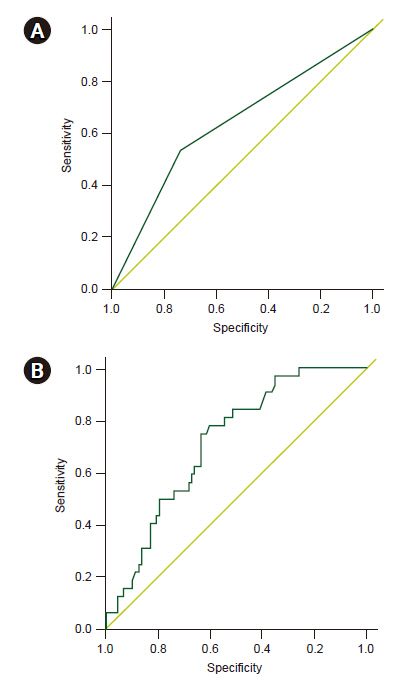
Pearson correlation showed that the cephalhematoma size in the pre-operative brain CT was weakly correlated with intraoperative blood loss (Pearson coefficient = 0.192, P = 0.037). In a multivariable logistic regression analysis, a cephalhematoma size greater than 113.5 cm3 was found to be a risk factor for large blood loss. The area under the curve in the receiver operating characteristic plot of the multivariable model was 0.714 (0.619–0.809).
Conclusions
A cephalhematoma size cutoff value of 113.5 cm3, as measured in the preoperative CT imaging, can predict intraoperative blood loss exceeding 30% of the total body blood volume. The establishment of a transfusion strategy prior to surgery based on cephalhematoma size could be useful in pediatric cranioplasty.
Figure
Reference
-
1. Liu L, Dong C, Chen L. Surgical treatment of ossified cephalhematoma: a case report and review of the literature. World Neurosurg. 2016; 96:614.e7–9.2. Chung HY, Chung JY, Lee DG, Yang JD, Baik BS, Hwang SG, et al. Surgical treatment of ossified cephalhematoma. J Craniofac Surg. 2004; 15:774–9.3. Guclu B, Yalcinkaya U, Kazanci B, Adilay U, Ekici MA. Diagnosis and treatment of ossified cephalhematoma. J Craniofac Surg. 2012; 23:e505–7.4. Gupta PK, Mathew GS, Malik AK, Al Derazi T. Ossified cephalhematoma. Pediatr Neurosurg. 2007; 43:492–7.5. Rothermel LD, Lipman JM. Estimation of blood loss is inaccurate and unreliable. Surgery. 2016; 160:946–53.6. Goobie SM, Haas T. Bleeding management for pediatric craniotomies and craniofacial surgery. Paediatr Anaesth. 2014; 24:678–89.7. Nafiu OO, Voepel-Lewis T, Morris M, Chimbira WT, Malviya S, Reynolds PI, et al. How do pediatric anesthesiologists define intraoperative hypotension? Paediatr Anaesth. 2009; 19:1048–53.8. Bhananker SM, Ramamoorthy C, Geiduschek JM, Posner KL, Domino KB, Haberkern CM, et al. Anesthesia-related cardiac arrest in children: update from the Pediatric Perioperative Cardiac Arrest Registry. Anesth Analg. 2007; 105:344–50.9. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012; 9:671–5.10. Dello SA, van Dam RM, Slangen JJ, van de Poll MC, Bemelmans MH, Greve JW, et al. Liver volumetry plug and play: do it yourself with ImageJ. World J Surg. 2007; 31:2215–21.11. Srinivasan L, Dutta R, Counsell SJ, Allsop JM, Boardman JP, Rutherford MA, et al. Quantification of deep gray matter in preterm infants at term-equivalent age using manual volumetry of 3-tesla magnetic resonance images. Pediatrics. 2007; 119:759–65.12. Rouette J, Trottier H, Ducruet T, Beaunoyer M, Lacroix J, Tucci M; Canadian Critical Care Trials Group; PALISI Network. Red blood cell transfusion threshold in postsurgical pediatric intensive care patients: a randomized clinical trial. Ann Surg. 2010; 251:421–7.13. Willems A, Harrington K, Lacroix J, Biarent D, Joffe AR, Wensley D, et al. TRIPICU investigators; Canadian Critical Care Trials Group; Pediatric Acute Lung Injury and Sepsis Investigators (PALISI) Network. Comparison of two red-cell transfusion strategies after pediatric cardiac surgery: a subgroup analysis. Crit Care Med. 2010; 38:649–56.14. Stainsby D, MacLennan S, Thomas D, Isaac J, Hamilton PJ; British Committee for Standards in Haematology. Guidelines on the management of massive blood loss. Br J Haematol. 2006; 135:634–41.15. Kozek-Langenecker SA, Afshari A, Albaladejo P, Santullano CA, De Robertis E, Filipescu DC, et al. Management of severe perioperative bleeding: guidelines from the European Society of Anaesthesiology. Eur J Anaesthesiol. 2013; 30:270–382.16. American College of Surgeons. Advanced trauma life support (ATLS): student course manual. 9th ed. Chicago: American College of Surgeons;2012.17. Cannon JW. Hemorrhagic shock. N Engl J Med. 2018; 378:1852–3.18. Diab YA, Wong EC, Luban NL. Massive transfusion in children and neonates. Br J Haematol. 2013; 161:15–26.19. Gutierrez G, Reines HD, Wulf-Gutierrez ME. Clinical review: hemorrhagic shock. Crit Care. 2004; 8:373–81.20. Zielinski MD, Wilson GA, Johnson PM, Polites SF, Jenkins DH, Harmsen WS, et al. Ideal hemoglobin transfusion target for resuscitation of massive-transfusion patients. Surgery. 2016; 160:1560–7.21. Lavoie J. Blood transfusion risks and alternative strategies in pediatric patients. Paediatr Anaesth. 2011; 21:14–24.22. Nguyen TT, Lam HV, Phillips M, Edwards C, Austin TM. Intraoperative optimization to decrease postoperative PRBC transfusion in children undergoing craniofacial reconstruction. Paediatr Anaesth. 2015; 25:294–300.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Spontaneous Rapid Resolution of Acute Epidural Hematoma in a Neonate
- Indications for the Diagnostic Tap of Cephalhematoma: A Survey of Case Reports
- Congenital Hemophilia B, Rare Mutation c.368T>G, Presenting with Delayed Massive Expansion of Non-Traumatic Cephalhema toma
- Multiple Cerebral Hemorrhages Caused by Paradoxical Reperfusion Injury After Cranioplasty
- Clinical Analysis of Epidural Fluid Collection as a Complication after Cranioplasty