Endocrinol Metab.
2021 Apr;36(2):374-387. 10.3803/EnM.2020.818.
Efficacy and Safety of the Novel Dipeptidyl Peptidase-4 Inhibitor Gemigliptin in the Management of Type 2 Diabetes: A Meta-Analysis
- Affiliations
-
- 1Department of Endocrinology, Center for Endocrinology, Diabetes, Arthritis & Rheumatism (CEDAR) Superspeciality Clinics, New Delhi, India
- 2Department of Endocrinology, Dr Ram Manohar Lohia (RML) Hospital, New Delhi, India
- 3Department of Endocrinology, R G Kar Medical College, Calcutta, India
- 4Department of Endocrinology, Kalpavriksh Healthcare, Dwarka, India
- 5Department of Endocrinology, Maharaja Agrasen Hospital, New Delhi, India
- 6Department of Rheumatology, Center for Endocrinology, Diabetes, Arthritis & Rheumatism (CEDAR) Superspeciality Clinics, New Delhi, India
- KMID: 2515464
- DOI: http://doi.org/10.3803/EnM.2020.818
Abstract
- Background
No meta-analysis has holistically analysed and summarised the efficacy and safety of gemigliptin in type 2 diabetes. The meta-analysis addresses this knowledge gap.
Methods
Electronic databases were searched for randomised controlled trials (RCTs) involving diabetes patients receiving gemigliptin in the intervention arm and placebo/active comparator in the control arm. The primary outcome was change in haemoglobin A1c (HbA1c). The secondary outcomes were alterations in glucose, glycaemic targets, lipids, insulin resistance, and adverse events.
Results
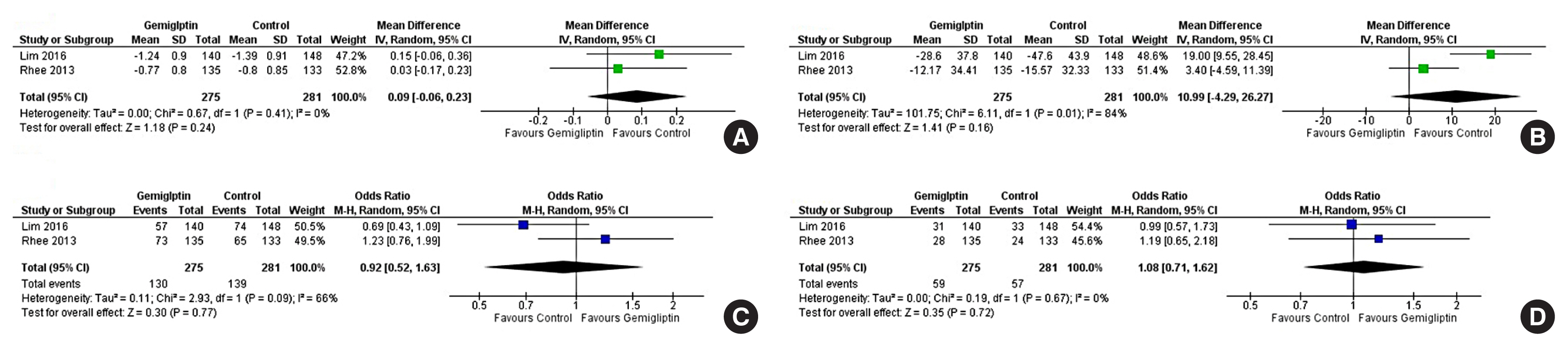
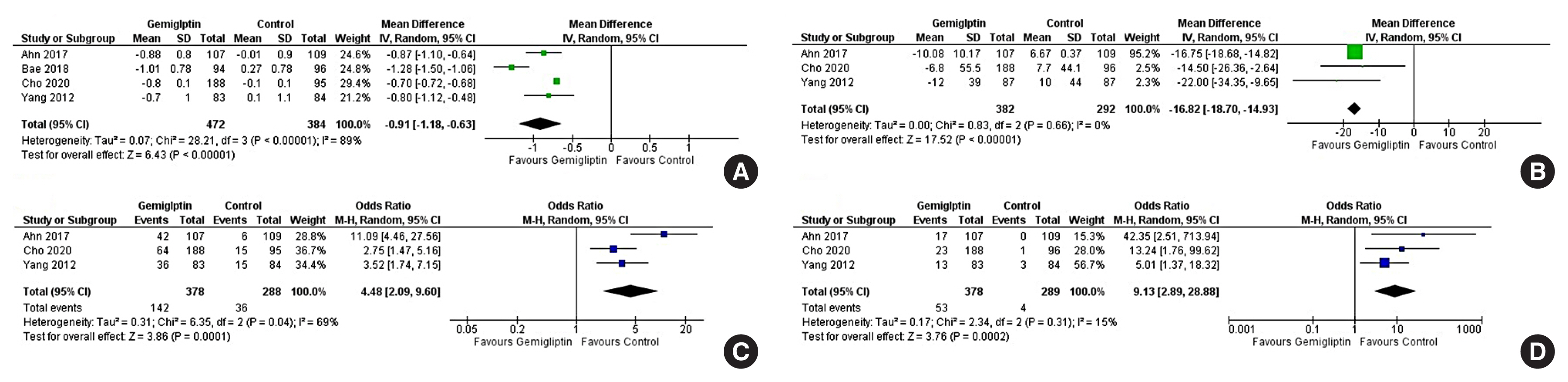
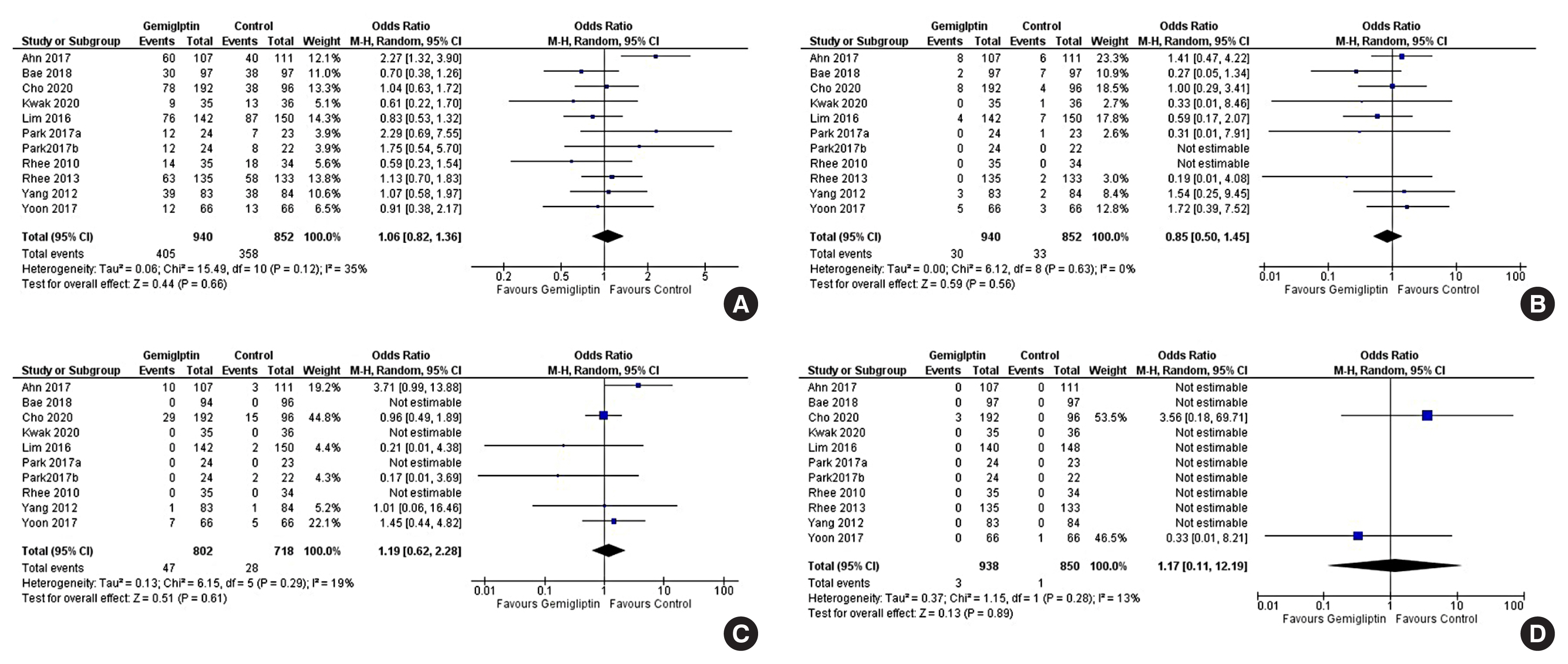
Data from 10 RCTs involving 1,792 patients were analysed. Four had an active control group (ACG), with metformin/dapagliflozin/sitagliptin/glimepiride as the active comparator; six had a passive control group (PCG), with placebo/rosuvastatin as controls. HbA1c reduction by gemigliptin at 24 weeks was comparable to ACG (mean difference [MD], 0.09%; 95% confidence interval [CI], –0.06 to 0.23; P=0.24; I2=0%; moderate certainty of evidence [MCE]), but superior to PCG (MD, –0.91%; 95% CI, –1.18 to –0.63); P<0.01; I2=89%; high certainty of evidence [HCE]). Gemigliptin was superior to PCG regarding achieving HbA1c <7% (12 weeks: odds ratio [OR], 5.91; 95% CI, 1.34 to 26.08; P=0.02; I2=74%; 24 weeks: OR, 4.48; 95% CI, 2.09 to 9.60; P<0.01; I2=69%; HCE). Gemigliptin was comparable to ACG regarding achieving HbA1c <7% after 24 weeks (OR, 0.92; 95% CI, 0.52 to 1.63; P=0.77; I2=66%; MCE). Adverse events were similar between the gemigliptin and control groups (risk ratio [RR], 1.06; 95% CI, 0.82 to 1.36; P=0.66; I2=35%; HCE). The gemigliptin group did not have increased hypoglycaemia (RR, 1.19; 95% CI, 0.62 to 2.28; P=0.61; I2=19%; HCE).
Conclusion
Gemigliptin has good glycaemic efficacy and is well-tolerated over 6 months of use.
Keyword
Figure
Cited by 1 articles
-
Safety and tolerability of sodium-glucose cotransporter-2 inhibitors in children and young adults: a systematic review and meta-analysis
Lakshmi Nagendra, Deep Dutta, Harish Bukkasagar Girijashankar, Deepak Khandelwal, Tejal Lathia, Meha Sharma
Ann Pediatr Endocrinol Metab. 2024;29(2):82-89. doi: 10.6065/apem.2346162.081.
Reference
-
1. Kim SH, Yoo JH, Lee WJ, Park CY. Gemigliptin: an update of its clinical use in the management of type 2 diabetes mellitus. Diabetes Metab J. 2016; 40:339–53.
Article2. Kim SH, Jung E, Yoon MK, Kwon OH, Hwang DM, Kim DW, et al. Pharmacological profiles of gemigliptin (LC15-0444), a novel dipeptidyl peptidase-4 inhibitor, in vitro and in vivo. Eur J Pharmacol. 2016; 788:54–64.
Article3. Kim N, Patrick L, Mair S, Stevens L, Ford G, Birks V, et al. Absorption, metabolism and excretion of [14C]gemigliptin, a novel dipeptidyl peptidase 4 inhibitor, in humans. Xenobiotica. 2014; 44:522–30.
Article4. Kim Y, Kim U, Kim IS, Lee SH, Lee J, Kim DH, et al. Absorption, distribution, metabolism and excretion of gemigliptin, a novel dipeptidyl peptidase IV inhibitor, in rats. Xenobiotica. 2014; 44:627–34.
Article5. Shon JH, Kim N, Park SJ, Oh MK, Kim EY, Lee SH, et al. Effect of renal impairment and haemodialysis on the pharmacokinetics of gemigliptin (LC15-0444). Diabetes Obes Metab. 2014; 16:1028–31.
Article6. Gutch M, Joshi A, Kumar S, Agarwal A, Pahan RK, Razi SM. Gemigliptin: newer promising gliptin for type 2 diabetes mellitus. Indian J Endocrinol Metab. 2017; 21:898–902.
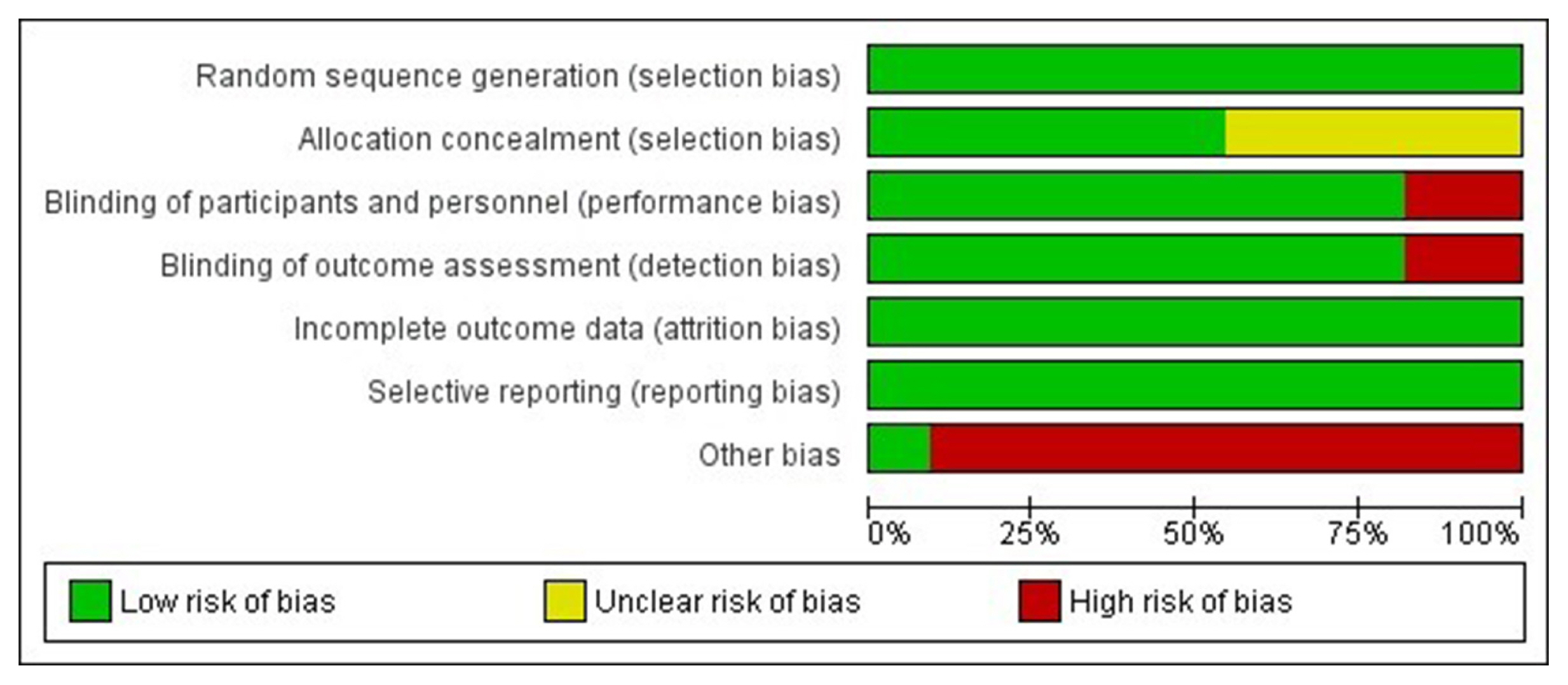
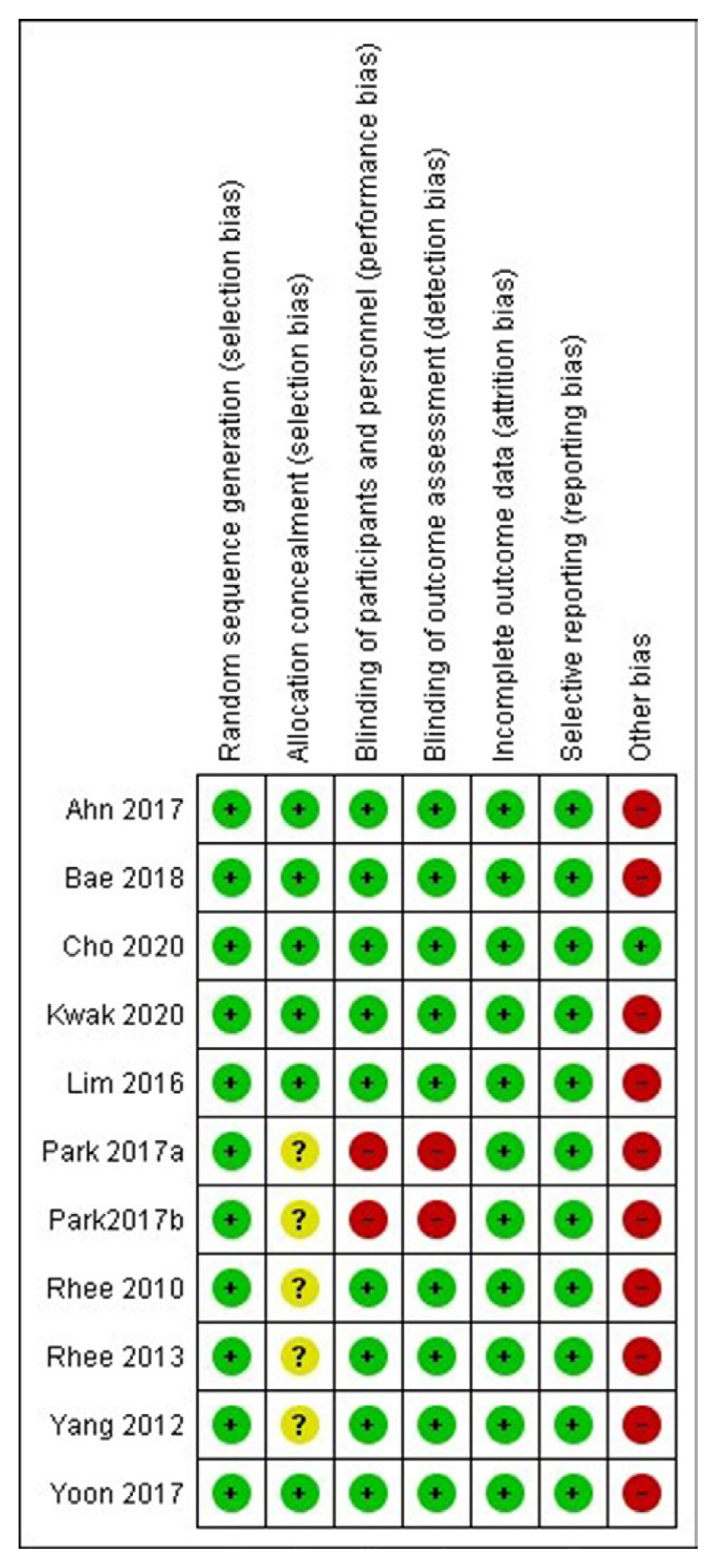
Article7. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article8. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009; 339:b2700.
Article9. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008; 336:924–6.
Article10. Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000; 4:1–115.
Article11. Kwak SH, Hwang YC, Won JC, Bae JC, Kim HJ, Suh S, et al. Comparison of the effects of gemigliptin and dapagliflozin on glycaemic variability in type 2 diabetes: a randomized, open-label, active-controlled, 12-week study (STABLE II study). Diabetes Obes Metab. 2020; 22:173–81.
Article12. Lim S, Han KA, Yu J, Chamnan P, Kim ES, Yoon KH, et al. Efficacy and safety of initial combination therapy with gemigliptin and metformin compared with monotherapy with either drug in patients with type 2 diabetes: a double-blind randomized controlled trial (INICOM study). Diabetes Obes Metab. 2017; 19:87–97.
Article13. Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, Jang HC, et al. Efficacy and safety of the dipeptidyl peptidase-4 inhibitor gemigliptin compared with sitagliptin added to ongoing metformin therapy in patients with type 2 diabetes inadequately controlled with metformin alone. Diabetes Obes Metab. 2013; 15:523–30.
Article14. Park SE, Lee BW, Kim JH, Lee WJ, Cho JH, Jung CH, et al. Effect of gemigliptin on glycaemic variability in patients with type 2 diabetes (STABLE study). Diabetes Obes Metab. 2017; 19:892–6.
Article15. Ahn CH, Han KA, Yu JM, Nam JY, Ahn KJ, Oh TK, et al. Efficacy and safety of gemigliptin, a dipeptidyl peptidase-4 inhibitor, in patients with type 2 diabetes mellitus inadequately controlled with combination treatment of metformin and sulphonylurea: a 24-week, multicentre, randomized, double-blind, placebo-controlled study (TROICA study). Diabetes Obes Metab. 2017; 19:635–43.
Article16. Cho YM, Deerochanawong C, Seekaew S, Suraamornkul S, Benjachareonwong S, Sattanon S, et al. Efficacy and safety of gemigliptin as add-on therapy to insulin, with or without metformin, in patients with type 2 diabetes mellitus (ZEUS II study). Diabetes Obes Metab. 2020; 22:123–7.
Article17. Rhee EJ, Lee WY, Yoon KH, Yoo SJ, Lee IK, Baik SH, et al. A multicenter, randomized, placebo-controlled, double-blind phase II trial evaluating the optimal dose, efficacy and safety of LC 15-0444 in patients with type 2 diabetes. Diabetes Obes Metab. 2010; 12:1113–9.
Article18. Yang SJ, Min KW, Gupta SK, Park JY, Shivane VK, Pitale SU, et al. A multicentre, multinational, randomized, placebo-controlled, double-blind, phase 3 trial to evaluate the efficacy and safety of gemigliptin (LC15-0444) in patients with type 2 diabetes. Diabetes Obes Metab. 2013; 15:410–6.
Article19. Yoon SA, Han BG, Kim SG, Han SY, Jo YI, Jeong KH, et al. Efficacy, safety and albuminuria-reducing effect of gemigliptin in Korean type 2 diabetes patients with moderate to severe renal impairment: a 12-week, double-blind randomized study (the GUARD Study). Diabetes Obes Metab. 2017; 19:590–8.
Article20. Bae JC, Min KW, Kim YH, Kim KA, Hong EG, Park CY, et al. Efficacy and safety of fixed-dose combination therapy with gemigliptin (50 mg) and rosuvastatin compared with monotherapy in patients with type 2 diabetes and dyslipidaemia (BALANCE): a multicentre, randomized, double-blind, controlled, phase 3 trial. Diabetes Obes Metab. 2019; 21:103–11.
Article21. Han SY, Yoon SA, Han BG, Kim SG, Jo YI, Jeong KH, et al. Comparative efficacy and safety of gemigliptin versus linagliptin in type 2 diabetes patients with renal impairment: a 40-week extension of the GUARD randomized study. Diabetes Obes Metab. 2018; 20:292–300.
Article22. Jung CH, Rhee EJ, Lee WY, Min KW, Shivane VK, Sosale AR, et al. A 52-week extension study of switching from gemigliptin vs sitagliptin to gemigliptin only as add-on therapy for patients with type 2 diabetes who are inadequately controlled with metformin alone. Diabetes Obes Metab. 2018; 20:1535–41.
Article23. Cha SA, Park YM, Yun JS, Lim TS, Song KH, Yoo KD, et al. A comparison of effects of DPP-4 inhibitor and SGLT2 inhibitor on lipid profile in patients with type 2 diabetes. Lipids Health Dis. 2017; 16:58.
Article24. Ampudia-Blasco FJ, Ceriello A. Importance of daily glycemic variability in achieving glycemic targets in type 2 diabetes: role of DPP-4 inhibitors. Med Clin (Barc). 2010; 135(Suppl 2):33–9.25. Liu X, Men P, Wang B, Cai G, Zhao Z. Effect of dipeptidyl-peptidase-4 inhibitors on C-reactive protein in patients with type 2 diabetes: a systematic review and meta-analysis. Lipids Health Dis. 2019; 18:144.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Gemigliptin: An Update of Its Clinical Use in the Management of Type 2 Diabetes Mellitus
- Emerging Safety Issues of Dipeptidyl Peptidase-4 Inhibitors and Sodium Glucose Cotransporter 2 Inhibitors: How to Interpret and Apply in Clinical Practice
- Effect of Dipeptidyl Peptidase-4 Inhibitors on Cardiovascular Outcome
- Dipeptidyl Peptidase 4 Inhibitor, an Update
- Novel Therapies for Type 2 Diabetes Mellitus