J Korean Med Sci.
2021 Apr;36(16):e102. 10.3346/jkms.2021.36.e102.
Optimal Volume of the Residual Tumor to Predict Long-term Tumor Control Using Stereotactic Radiosurgery after Facial Nerve-preserving Surgery for Vestibular Schwannomas
- Affiliations
-
- 1Department of Neurosurgery, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Otorhinolaryngology, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- KMID: 2515084
- DOI: http://doi.org/10.3346/jkms.2021.36.e102
Abstract
- Background
Intended subtotal resection (STR) followed by adjuvant gamma knife radiosurgery (GKRS) has emerged as an effective treatment option for facial nerve (FN) preservation in vestibular schwannomas (VSs). This study aimed to identify the optimal cutoff volume of residual VS to predict favorable outcomes in terms of both tumor control and FN preservation.
Methods
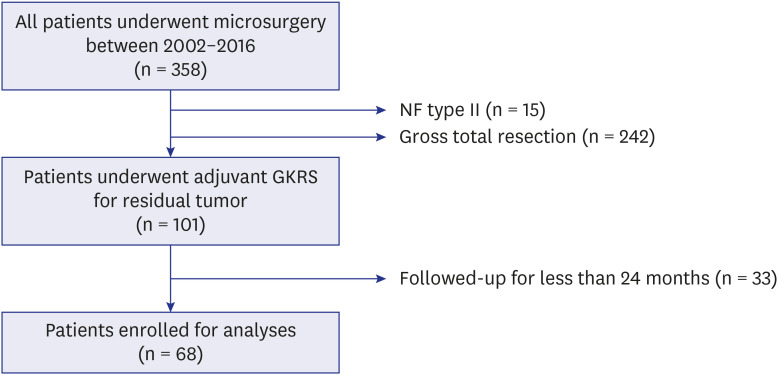
This retrospective study assessed the patients who underwent adjuvant GKRS for residual VS after microsurgery. A total of 68 patients who had been followed up for ≥ 24 months after GKRS were included. Tumor progression was defined as an increase in tumor volume (TV) of ≥ 20%. House-Brackmann grades I and II were considered to indicate good FN function.
Results
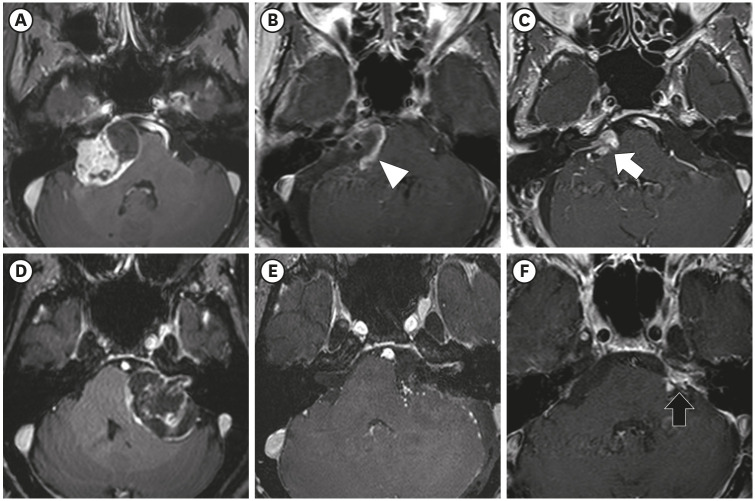
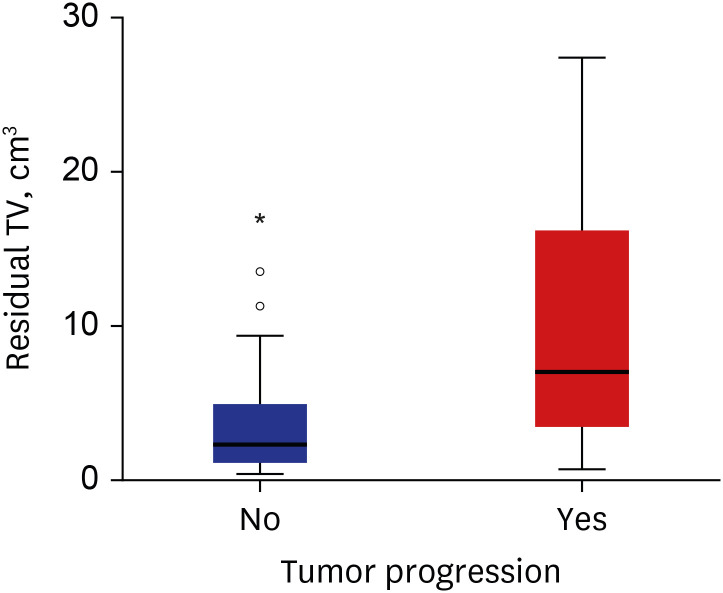
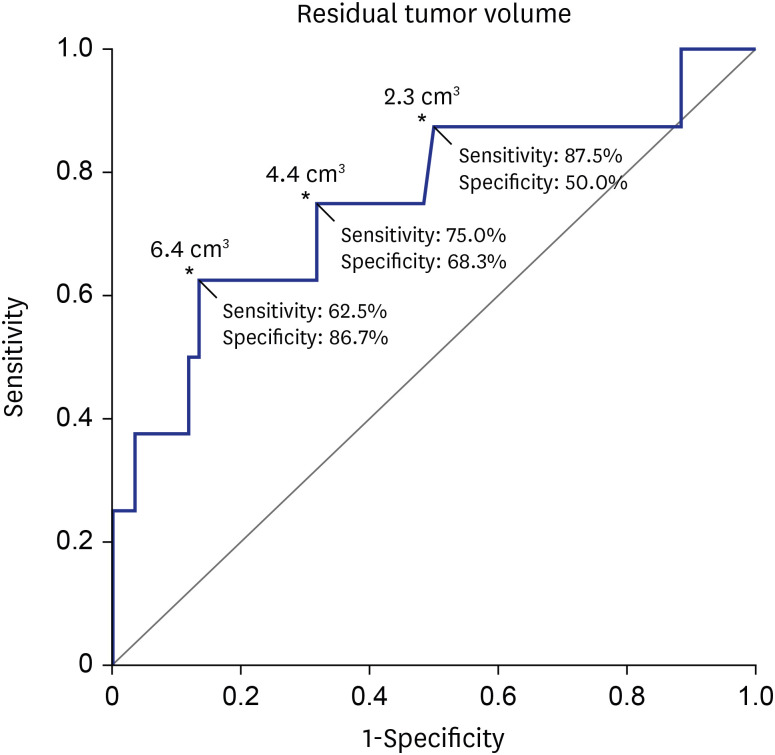
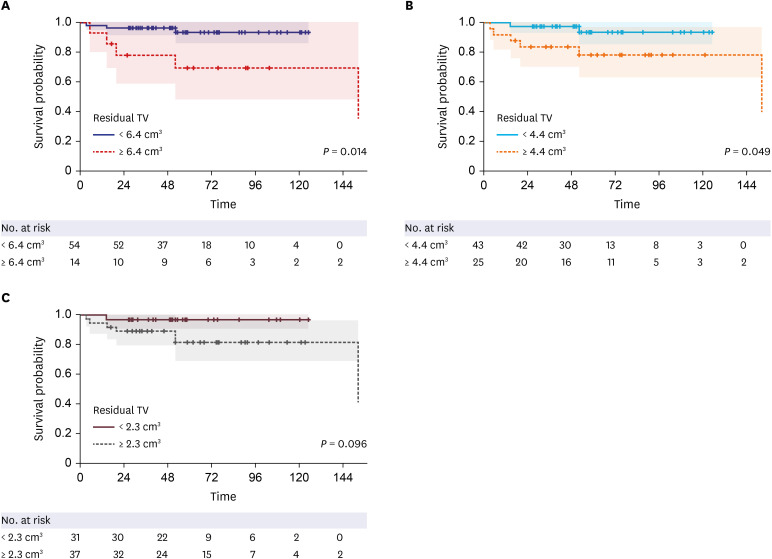
The median residual TV was 2.5 cm3 (range: 0.3–27.4). The median follow-up period after the first adjuvant GKRS was 64 months (range: 25.7–152.4). Eight (12%) patients showed tumor progression. In multivariate analyses, residual TV was associated with tumor progression (P = 0.003; hazard ratio [HR], 1.229; 95% confidence interval [CI], 1.075–1.405). A residual TV of 6.4 cm3 was identified as the cut-off volume for showing the greatest difference in progression-free survival (PFS). The 5-year PFS rates in the group with residual TVs of < 6.4 cm3 (54 patients) and that with residual TVs of ≥ 6.4 cm3 (14 patients) were 93.3% and 69.3%, respectively (P = 0.014). A good FN outcome was achieved in 57 (84%) patients. Residual TV was not associated with good FN function during the immediate postoperative period (P = 0.695; odds ratio [OR], 1.024; 95% CI, 0.908–1.156) or at the last follow-up (P = 0.755; OR, 0.980; 95% CI, 0.866–1.110).
Conclusion
In this study, residual TV was associated with tumor progression in VS after adjuvant GKRS following STR. As preservation of FN function is not correlated with the extent of resection, optimal volume reduction is imperative to achieve long-term tumor control. Our findings will help surgeons predict the prognosis of residual VS after FNpreserving surgery.
Figure
Reference
-
1. Stangerup SE, Caye-Thomasen P. Epidemiology and natural history of vestibular schwannomas. Otolaryngol Clin North Am. 2012; 45(2):257–268. PMID: 22483814.
Article2. Betchen SA, Walsh J, Post KD. Self-assessed quality of life after acoustic neuroma surgery. J Neurosurg. 2003; 99(5):818–823. PMID: 14609159.
Article3. Bloch DC, Oghalai JS, Jackler RK, Osofsky M, Pitts LH. The fate of the tumor remnant after less-than-complete acoustic neuroma resection. Otolaryngol Head Neck Surg. 2004; 130(1):104–112. PMID: 14726918.
Article4. Chen Z, Prasad SC, Di Lella F, Medina M, Piccirillo E, Taibah A, et al. The behavior of residual tumors and facial nerve outcomes after incomplete excision of vestibular schwannomas. J Neurosurg. 2014; 120(6):1278–1287. PMID: 24724851.
Article5. Nakatomi H, Jacob JT, Carlson ML, Tanaka S, Tanaka M, Saito N, et al. Long-term risk of recurrence and regrowth after gross-total and subtotal resection of sporadic vestibular schwannoma. J Neurosurg. Forthcoming. 2017; DOI: 10.3171/2016.11.JNS16498.
Article6. Seol HJ, Kim CH, Park CK, Kim CH, Kim DG, Chung YS, et al. Optimal extent of resection in vestibular schwannoma surgery: relationship to recurrence and facial nerve preservation. Neurol Med Chir (Tokyo). 2006; 46(4):176–180. PMID: 16636507.
Article7. Schwartz MS, Kari E, Strickland BM, Berliner K, Brackmann DE, House JW, et al. Evaluation of the increased use of partial resection of large vestibular schwanommas: facial nerve outcomes and recurrence/regrowth rates. Otol Neurotol. 2013; 34(8):1456–1464. PMID: 23928516.8. Bernardeschi D, Pyatigorskaya N, Vanier A, Bielle F, Smail M, Lamas G, et al. Role of electrophysiology in guiding near-total resection for preservation of facial nerve function in the surgical treatment of large vestibular schwannomas. J Neurosurg. 2018; 128(3):903–910. PMID: 28409723.
Article9. van de Langenberg R, Hanssens PE, van Overbeeke JJ, Verheul JB, Nelemans PJ, de Bondt BJ, et al. Management of large vestibular schwannoma. Part I. Planned subtotal resection followed by gamma knife surgery: radiological and clinical aspects. J Neurosurg. 2011; 115(5):875–884. PMID: 21838510.
Article10. Bailo M, Boari N, Gagliardi F, Franzin A, Piloni M, Spina A, et al. Gamma knife radiosurgery for residual and recurrent vestibular schwannomas after previous surgery: clinical results in a series of 90 patients and review of the literature. World Neurosurg. 2017; 98:60–72. PMID: 27777157.
Article11. Zumofen DW, Guffi T, Epple C, Westermann B, Krähenbühl AK, Zabka S, et al. Intended near-total removal of Koos grade IV vestibular schwannomas: reconsidering the treatment paradigm. Neurosurgery. 2018; 82(2):202–210. PMID: 28383680.
Article12. Brokinkel B, Sauerland C, Holling M, Ewelt C, Horstmann G, van Eck AT, et al. Gamma knife radiosurgery following subtotal resection of vestibular schwannoma. J Clin Neurosci. 2014; 21(12):2077–2082. PMID: 25065850.
Article13. Iwai Y, Ishibashi K, Watanabe Y, Uemura G, Yamanaka K. Functional Preservation after planned partial resection followed by gamma knife radiosurgery for large vestibular schwannomas. World Neurosurg. 2015; 84(2):292–300. PMID: 25790872.
Article14. Daniel RT, Tuleasca C, George M, Pralong E, Schiappacasse L, Zeverino M, et al. Preserving normal facial nerve function and improving hearing outcome in large vestibular schwannomas with a combined approach: planned subtotal resection followed by gamma knife radiosurgery. Acta Neurochir (Wien). 2017; 159(7):1197–1211. PMID: 28516364.
Article15. Starnoni D, Daniel RT, Tuleasca C, George M, Levivier M, Messerer M. Systematic review and meta-analysis of the technique of subtotal resection and stereotactic radiosurgery for large vestibular schwannomas: a “nerve-centered” approach. Neurosurg Focus. 2018; 44(3):E4.
Article16. Bretonnier M, Bernard F, Tinois J, Troude L, Cebula H, Godey B, et al. Functional sparing surgery policy for giant vestibular schwannomas. Clin Otolaryngol. 2020; 45(5):762–767. PMID: 32449573.
Article17. Haque R, Wojtasiewicz TJ, Gigante PR, Attiah MA, Huang B, Isaacson SR, et al. Efficacy of facial nerve-sparing approach in patients with vestibular schwannomas. J Neurosurg. 2011; 115(5):917–923. PMID: 21854113.
Article18. Monfared A, Corrales CE, Theodosopoulos PV, Blevins NH, Oghalai JS, Selesnick SH, et al. Facial nerve outcome and tumor control rate as a function of degree of resection in treatment of large acoustic neuromas: preliminary report of the Acoustic Neuroma Subtotal Resection Study (ANSRS). Neurosurgery. 2016; 79(2):194–203. PMID: 26645964.19. Suero Molina E, van Eck AT, Sauerland C, Schipmann S, Horstmann G, Stummer W, et al. Local tumor control and clinical symptoms after gamma knife radiosurgery for residual and recurrent vestibular schwannomas. World Neurosurg. 2019; 122:e1240–6. PMID: 30447443.
Article20. Iwai Y, Yamanaka K, Ishiguro T. Surgery combined with radiosurgery of large acoustic neuromas. Surg Neurol. 2003; 59(4):283–289. PMID: 12748011.
Article21. Radwan H, Eisenberg MB, Sandberg Knisely JP, Ghaly MM, Schulder M. Outcomes in patients with vestibular schwannoma after subtotal resection and adjuvant radiosurgery. Stereotact Funct Neurosurg. 2016; 94(4):216–224. PMID: 27513938.
Article22. Starnoni D, Giammattei L, Cossu G, Link MJ, Roche PH, Chacko AG, et al. Surgical management for large vestibular schwannomas: a systematic review, meta-analysis, and consensus statement on behalf of the EANS skull base section. Acta Neurochir (Wien). 2020; 162(11):2595–2617. PMID: 32728903.
Article23. Yang SY, Kim DG, Chung HT, Park SH, Paek SH, Jung HW. Evaluation of tumour response after gamma knife radiosurgery for residual vestibular schwannomas based on MRI morphological features. J Neurol Neurosurg Psychiatry. 2008; 79(4):431–436. PMID: 17673492.
Article24. Breshears JD, Morshed RA, Molinaro AM, McDermott MW, Cheung SW, Theodosopoulos PV. Residual tumor volume and location predict progression after primary subtotal resection of sporadic vestibular schwannomas: a retrospective volumetric study. Neurosurgery. 2020; 86(3):410–416. PMID: 31232426.
Article25. Kim KH, Cho YS, Seol HJ, Cho KR, Choi JW, Kong DS, et al. Comparison between retrosigmoid and translabyrinthine approaches for large vestibular schwannoma: focus on cerebellar injury and morbidities. Neurosurg Rev. 2021; 44(1):351–361. PMID: 31758338.
Article26. Shao KN, Tatagiba M, Samii M. Surgical management of high jugular bulb in acoustic neurinoma via retrosigmoid approach. Neurosurgery. 1993; 32(1):32–36. PMID: 8421554.
Article27. Tatagiba M, Roser F, Schuhmann MU, Ebner FH. Vestibular schwannoma surgery via the retrosigmoid transmeatal approach. Acta Neurochir (Wien). 2014; 156(2):421–425. PMID: 24292774.
Article28. Koval J, Molcan M, Bowdler AD, Sterkers JM. Retrosigmoid transmeatal approach: an anatomic study of an approach used for preservation of hearing in acoustic neuroma surgery and vestibular neurotomy. Skull Base Surg. 1993; 3(1):16–21. PMID: 17170885.
Article29. Day JD, Kellogg JX, Fukushima T, Giannotta SL. Microsurgical anatomy of the inner surface of the petrous bone: neuroradiological and morphometric analysis as an adjunct to the retrosigmoid transmeatal approach. Neurosurgery. 1994; 34(6):1003–1008. PMID: 8084384.30. González-Darder JM, Capilla-Guasch P, Escartín FP. Magnetic resonance imaging surveillance for vestibular schwannoma after microsurgical resection using a retrosigmoid transmeatal approach. World Neurosurg. 2020; 139:e585–91. PMID: 32371074.
Article31. van de Langenberg R, de Bondt BJ, Nelemans PJ, Baumert BG, Stokroos RJ. Follow-up assessment of vestibular schwannomas: volume quantification versus two-dimensional measurements. Neuroradiology. 2009; 51(8):517–524. PMID: 19418046.
Article32. Hayhurst C, Zadeh G. Tumor pseudoprogression following radiosurgery for vestibular schwannoma. Neuro-oncol. 2012; 14(1):87–92. PMID: 22028389.
Article33. Meijer OW, Weijmans EJ, Knol DL, Slotman BJ, Barkhof F, Vandertop WP, et al. Tumor-volume changes after radiosurgery for vestibular schwannoma: implications for follow-up MR imaging protocol. AJNR Am J Neuroradiol. 2008; 29(5):906–910. PMID: 18296549.
Article34. House JW, Brackmann DE. Facial nerve grading system. Otolaryngol Head Neck Surg. 1985; 93(2):146–147. PMID: 3921901.
Article35. Gardner G, Robertson JH. Hearing preservation in unilateral acoustic neuroma surgery. Ann Otol Rhinol Laryngol. 1988; 97(1):55–66. PMID: 3277525.
Article36. Zhang S, Liu W, Hui X, You C. Surgical treatment of giant vestibular schwannomas: facial nerve outcome and tumor control. World Neurosurg. 2016; 94:137–144. PMID: 27392890.
Article37. Goldbrunner R, Weller M, Regis J, Lund-Johansen M, Stavrinou P, Reuss D, et al. EANO guideline on the diagnosis and treatment of vestibular schwannoma. Neuro-oncol. 2020; 22(1):31–45. PMID: 31504802.
Article38. Vakilian S, Souhami L, Melançon D, Zeitouni A. Volumetric measurement of vestibular schwannoma tumour growth following partial resection: predictors for recurrence. J Neurol Surg B Skull Base. 2012; 73(2):117–120. PMID: 23542125.
Article39. Sheehan J, Lee CC, Bodach ME, Tumialan LM, Oyesiku NM, Patil CG, et al. Congress of neurological surgeons systematic review and evidence-based guideline for the management of patients with residual or recurrent nonfunctioning pituitary adenomas. Neurosurgery. 2016; 79(4):E539–40. PMID: 27635963.
Article40. Troude L, Boucekine M, Montava M, Lavieille JP, Régis JM, Roche PH. Predictive factors of early postoperative and long-term facial nerve function after large vestibular schwannoma surgery. World Neurosurg. 2019; 127:e599–608. PMID: 30930324.
Article41. Daoudi H, Lahlou G, Degos V, Sterkers O, Nguyen Y, Kalamarides M. Improving facial nerve outcome and hearing preservation by different degrees of vestibular schwannoma resection guided by intraoperative facial nerve electromyography. Acta Neurochir (Wien). 2020; 162(8):1983–1993. PMID: 32424567.
Article42. Lefranc M, Da Roz LM, Balossier A, Thomassin JM, Roche PH, Regis J. Place of gamma knife stereotactic radiosurgery in grade 4 vestibular schwannoma based on case series of 86 patients with long-term follow-up. World Neurosurg. 2018; 114:e1192–8. PMID: 29614352.
Article43. Watanabe S, Yamamoto M, Kawabe T, Koiso T, Aiyama H, Kasuya H, et al. Long-term follow-up results of stereotactic radiosurgery for vestibular schwannomas larger than 8 cc. Acta Neurochir (Wien). 2019; 161(7):1457–1465. PMID: 31127373.44. Peng KA, Chen BS, Lorenz MB, Lekovic GP, Schwartz MS, Slattery WH, et al. Revision surgery for vestibular schwannomas. J Neurol Surg B Skull Base. 2018; 79(6):528–532. PMID: 30456020.45. Aboukaïs R, Bonne NX, Touzet G, Vincent C, Reyns N, Lejeune JP. Progression of vestibular schawnnoma after GammaKnife radiosurgery: a challenge for microsurgical resection. Clin Neurol Neurosurg. 2018; 168:77–82. PMID: 29525732.
Article46. Wise SC, Carlson ML, Tveiten OV, Driscoll CL, Myrseth E, Lund-Johansen M, et al. Surgical salvage of recurrent vestibular schwannoma following prior stereotactic radiosurgery. Laryngoscope. 2016; 126(11):2580–2586. PMID: 27107262.
Article47. Yomo S, Arkha Y, Delsanti C, Roche PH, Thomassin JM, Régis J. Repeat gamma knife surgery for regrowth of vestibular schwannomas. Neurosurgery. 2009; 64(1):48–54. PMID: 19050660.
Article48. Romiyo P, Ng E, Dejam D, Ding K, Sheppard JP, Duong C, et al. Radiosurgery treatment is associated with improved facial nerve preservation versus repeat resection in recurrent vestibular schwannomas. Acta Neurochir (Wien). 2019; 161(7):1449–1456. PMID: 31129783.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Long-term Outcomes of Gamma Knife Stereotactic Radiosurgery of Vestibular Schwannomas
- Surgical Findings to Differentiate Between Facial Nerve Schwannoma and Vestibular Schwannoma
- Gamma Knife Radiosurgery for Vestibular Schwannomas
- Hearing Outcome after Gamma Knife Stereotactic Radiosurgery in Vestibular Schwannoma Patients with Serviceable Hearing
- A Case of Vestibular Schwannoma Treated with Revision Middle Cranial Fossa Approach to Preserve Facial Nerve Function and Hearing Ability