J Korean Med Sci.
2021 Apr;36(15):e94. 10.3346/jkms.2021.36.e94.
Bile Microbiota in Patients with Pigment Common Bile Duct Stones
- Affiliations
-
- 1Digestive Disease Center, Department of Internal Medicine, Inha University School of Medicine, Incheon, Korea
- 2Hecto Innovation Lab., Hecto Co., Ltd., Seoul, Korea
- KMID: 2515035
- DOI: http://doi.org/10.3346/jkms.2021.36.e94
Abstract
- Background
Common bile duct (CBD) stone is one of the most prevalent gastroenterological diseases, but the role played by biliary microbiota in the pathogenesis of CBD stones remains obscure. The aim of this study was to investigate the characteristics of the biliary tract core microbiome and its potential association with the formation of pigment stones.
Methods
Twenty-eight patients with biliary obstruction of various causes were enrolled. Thirteen had new-onset pigment CBD stone. Of the remaining 15, four had benign biliary stricture, four had gallbladder cancer, three had pancreatic cancer, 3 had distal CBD cancer, and one had hepatocellular carcinoma. Endoscopic retrograde cholangiopancreatography was used to collect bile samples for DNA extraction, 16S ribosomal RNA gene sequencing, and bile microbiota composition analysis.
Results
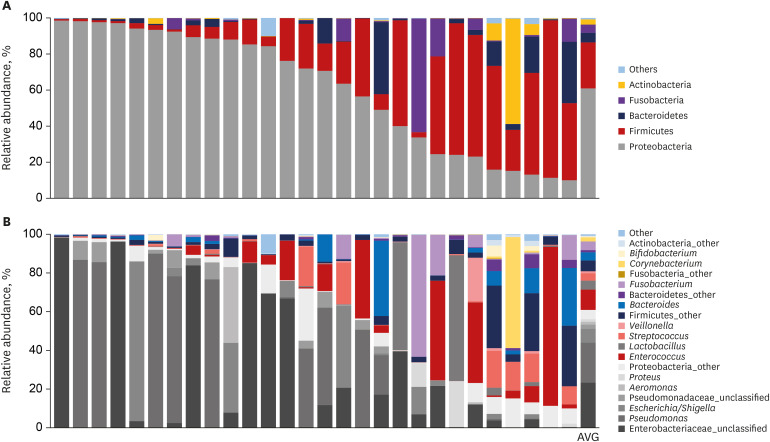
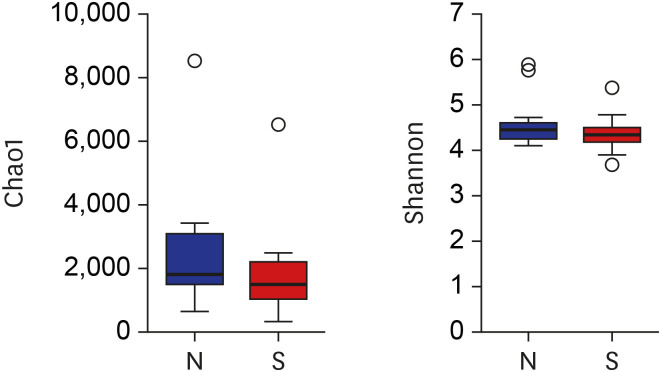
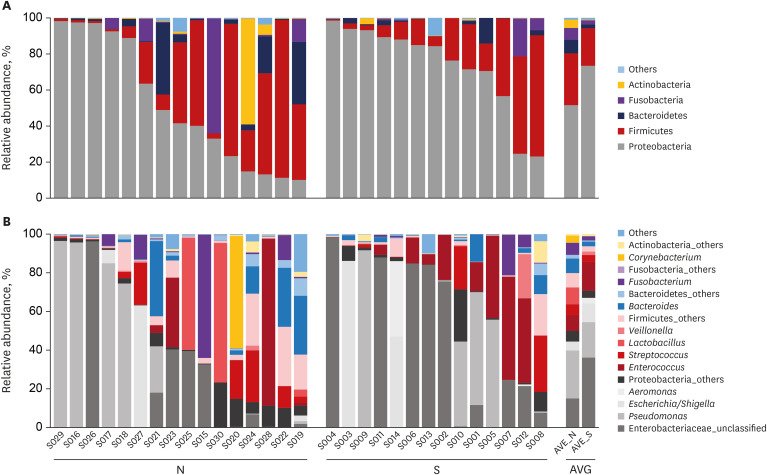
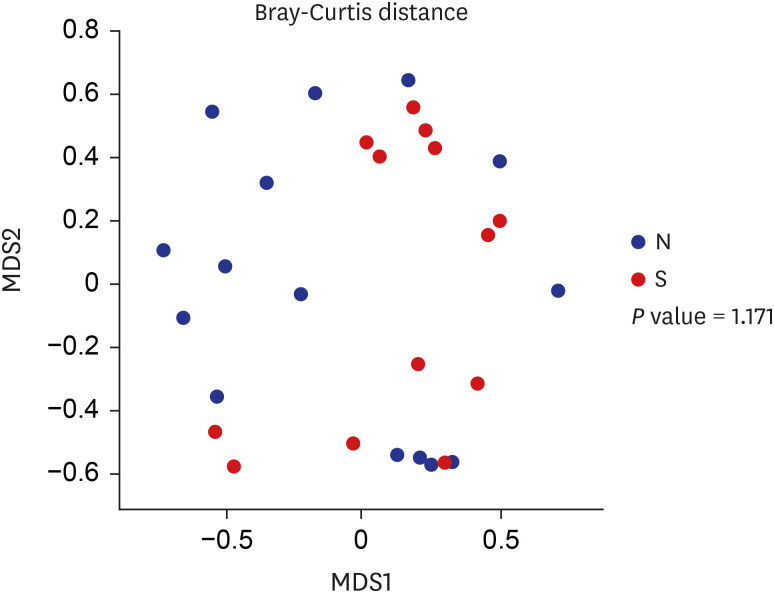
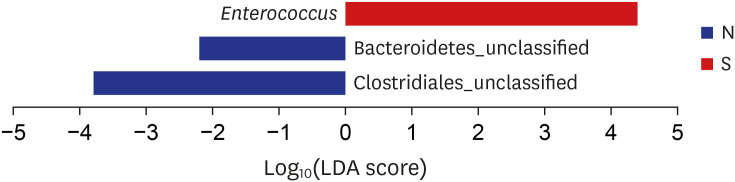
Proteobacteria (61.7%), Firmicutes (25.1%), Bacteroidetes (5%), Fusobacteria (4.6%), and Actinobacteria (2.6%) were the most dominant phyla in the bile of the 28 study subjects. A comparison between new-onset choledocholithiasis and other causes of biliary obstruction (controls) showed Enterococcus was found to be significantly abundant in the CBD stone group at the genus level (linear discriminant analysis score = 4.38; P = 0.03). However, no other significant compositional difference was observed.
Conclusion
This study demonstrates an abundance of microbiota in bile juice and presents a biliary microbiome composition similar to that of duodenum. The study also shows Enterococcus was significantly abundant in the bile juice of patients with a brown pigment stone than in controls, which suggests Enterococcus may play an important role in the development of pigment stones.
Keyword
Figure
Cited by 1 articles
-
Association of Microbial Dysbiosis with Gallbladder Diseases Identified by Bile Microbiome Profiling
Seong Ji Choi, Yeseul Kim, Jehyun Jeon, Ho-Jin Gwak, Mimi Kim, Kyojin Kang, Yohan Kim, Jaemin Jeong, Yun Kyung Jung, Kyeong Geun Lee, Ho Soon Choi, Dong-Hwan Jung, Sung-Gyu Lee, Yangsoon Lee, Su-Jin Shin, Kiseok Jang, Mina Rho, Dongho Choi
J Korean Med Sci. 2021;36(28):e189. doi: 10.3346/jkms.2021.36.e189.
Reference
-
1. Shaffer EA. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep. 2005; 7(2):132–140. PMID: 15802102.
Article2. Russo MW, Wei JT, Thiny MT, Gangarosa LM, Brown A, Ringel Y, et al. Digestive and liver diseases statistics, 2004. Gastroenterology. 2004; 126(5):1448–1453. PMID: 15131804.
Article3. Ebner S, Rechner J, Beller S, Erhart K, Riegler FM, Szinicz G. Laparoscopic management of common bile duct stones. Surg Endosc. 2004; 18(5):762–765. PMID: 14752631.
Article4. Velanovich V, Morton JM, McDonald M, Orlando R 3rd, Maupin G, Traverso LW, et al. Analysis of the SAGES outcomes initiative cholecystectomy registry. Surg Endosc. 2006; 20(1):43–50. PMID: 16333539.
Article5. Kim HG, Han J, Kim MH, Cho KH, Shin IH, Kim GH, et al. Prevalence of clonorchiasis in patients with gastrointestinal disease: a Korean nationwide multicenter survey. World J Gastroenterol. 2009; 15(1):86–94. PMID: 19115472.
Article6. Maki T. Pathogenesis of calcium bilirubinate gallstone: role of E. coli, beta-glucuronidase and coagulation by inorganic ions, polyelectrolytes and agitation. Ann Surg. 1966; 164(1):90–100. PMID: 5329901.7. Kaufman HS, Magnuson TH, Lillemoe KD, Frasca P, Pitt HA. The role of bacteria in gallbladder and common duct stone formation. Ann Surg. 1989; 209(5):584–591. PMID: 2705823.
Article8. Theron J, Cloete TE. Molecular techniques for determining microbial diversity and community structure in natural environments. Crit Rev Microbiol. 2000; 26(1):37–57. PMID: 10782339.
Article9. Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, et al. Experimental and analytical tools for studying the human microbiome. Nat Rev Genet. 2011; 13(1):47–58. PMID: 22179717.
Article10. Human Microbiome Project Consortium. Structure, function and diversity of the healthy human microbiome. Nature. 2012; 486(7402):207–214. PMID: 22699609.11. Gilbert JA, Lynch SV. Community ecology as a framework for human microbiome research. Nat Med. 2019; 25(6):884–889. PMID: 31133693.
Article12. Human Microbiome Project Consortium. A framework for human microbiome research. Nature. 2012; 486(7402):215–221. PMID: 22699610.13. Wu T, Zhang Z, Liu B, Hou D, Liang Y, Zhang J, et al. Gut microbiota dysbiosis and bacterial community assembly associated with cholesterol gallstones in large-scale study. BMC Genomics. 2013; 14(1):669. PMID: 24083370.
Article14. Shen H, Ye F, Xie L, Yang J, Li Z, Xu P, et al. Metagenomic sequencing of bile from gallstone patients to identify different microbial community patterns and novel biliary bacteria. Sci Rep. 2015; 5(1):17450. PMID: 26625708.
Article15. Schloss PD, Westcott SL, Ryabin T, Hall JR, Hartmann M, Hollister EB, et al. Introducing mothur: open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl Environ Microbiol. 2009; 75(23):7537–7541. PMID: 19801464.
Article16. R Core Team. R: a Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing;2013.17. Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992; 37(5):689–696. PMID: 1563308.
Article18. Schneider J, De Waha P, Hapfelmeier A, Feihl S, Römmler F, Schlag C, et al. Risk factors for increased antimicrobial resistance: a retrospective analysis of 309 acute cholangitis episodes. J Antimicrob Chemother. 2014; 69(2):519–525. PMID: 24084640.
Article19. Chen B, Fu SW, Lu L, Zhao H. A preliminary study of biliary microbiota in patients with bile duct stones or distal cholangiocarcinoma. BioMed Res Int. 2019; 2019:1092563. PMID: 31662965.
Article20. Jiménez E, Sánchez B, Farina A, Margolles A, Rodríguez JM. Characterization of the bile and gall bladder microbiota of healthy pigs. MicrobiologyOpen. 2014; 3(6):937–949. PMID: 25336405.
Article21. Kamada N, Chen GY, Inohara N, Núñez G. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol. 2013; 14(7):685–690. PMID: 23778796.
Article22. Aviles-Jimenez F, Vazquez-Jimenez F, Medrano-Guzman R, Mantilla A, Torres J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci Rep. 2014; 4(1):4202. PMID: 24569566.
Article23. Ye F, Shen H, Li Z, Meng F, Li L, Yang J, et al. Influence of the biliary system on biliary bacteria revealed by bacterial communities of the human biliary and upper digestive tracts. PLoS One. 2016; 11(3):e0150519. PMID: 26930491.
Article24. Nomura T, Shirai Y, Hatakeyama K. Enterococcal bactibilia in patients with malignant biliary obstruction. Dig Dis Sci. 2000; 45(11):2183–2186. PMID: 11215736.25. Lee YC, Song DK, Kim JS, Choi CS. Effect of cholestyramine on the formation of pigment gallstone in high carbohydrate diet-fed hamsters. J Korean Med Sci. 1996; 11(5):397–401. PMID: 8934394.
Article26. Skar V, Skar AG, Strømme JH. Beta-glucuronidase activity related to bacterial growth in common bile duct bile in gallstone patients. Scand J Gastroenterol. 1988; 23(1):83–90. PMID: 3344403.
Article27. Leung JW, Liu YL, Leung PS, Chan RC, Inciardi JF, Cheng AF. Expression of bacterial beta-glucuronidase in human bile: an in vitro study. Gastrointest Endosc. 2001; 54(3):346–350. PMID: 11522976.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Actinomycosis of the intrahepatic bile duct concomitant with intrahepatic duct stones
- A Comparative Study of Bile Compositions From patients with Gallbladder Stones and Common Bile Duct stones
- 4 Cases of Web of Common Bile Duct
- Application of Electrohydraulic Lithotripsy for Bile Duct Stones Difficult to Remove
- Concurrent Yellow-to-white and Black Extrahepatic Bile Duct Stones