Cancer Res Treat.
2021 Apr;53(2):436-444. 10.4143/crt.2020.725.
Trends in Chemotherapy Patterns and Survival of Patients with Advanced Gastric Cancer over a 16-Year Period: Impact of Anti-HER2–Targeted Agent in the Real-World Setting
- Affiliations
-
- 1Division of Hematology/Oncology, Department of Internal Medicine, Kangbuk Samsung Hospital, Sungkyunkwan University School of Medicine, Seoul, Korea
- 2Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- 3Division of Biostatistics, Department of R&D Management, Kangbuk Samsung Hospital, Seoul, Korea
- KMID: 2514925
- DOI: http://doi.org/10.4143/crt.2020.725
Abstract
- Purpose
This study aimed to evaluate the survivals of patients with metastatic or recurrent gastric cancer (MRGC) over a period of 16 years and to investigate the recent changes in chemotherapy patterns.
Materials and Methods
A total of 5,384 patients who received chemotherapy for MRGC between 2000 and 2015 were analyzed. The analysis focused on a comparison of the first-line chemotherapy between four periods: 2000–2003 (period 1), 2004–2007 (period 2), 2008–2011 (period 3), and 2012–2015 (period 4).
Results
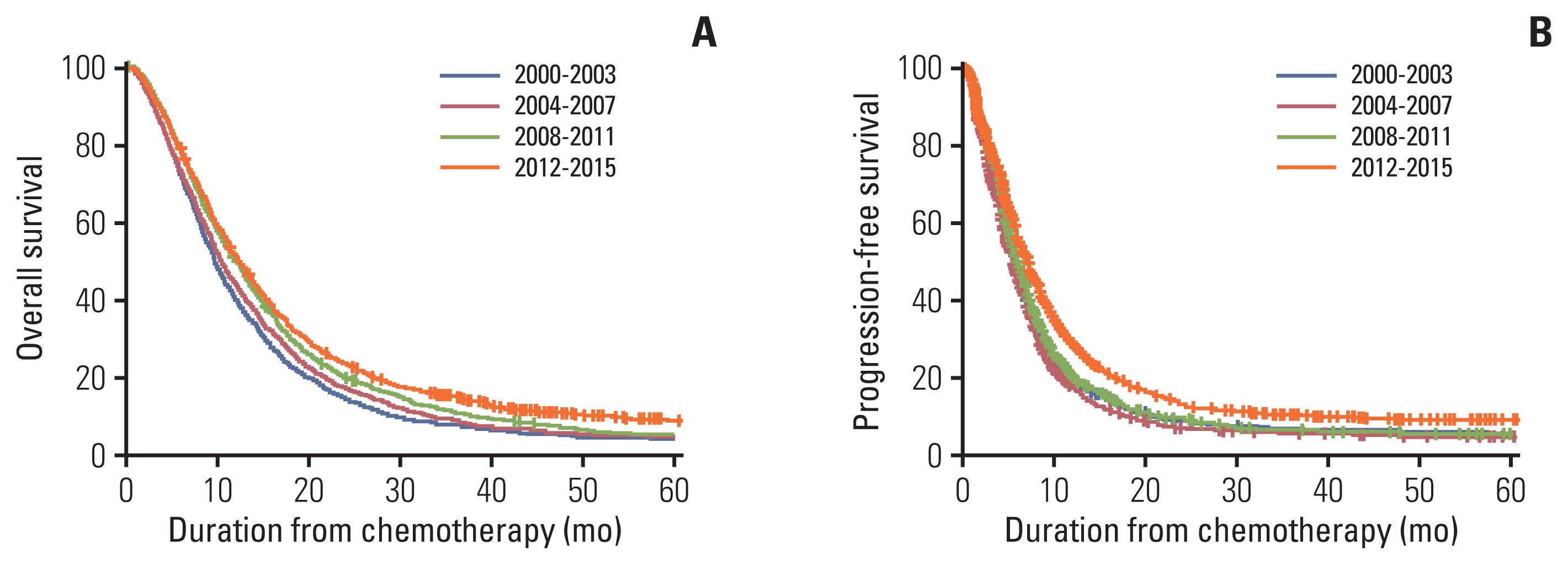
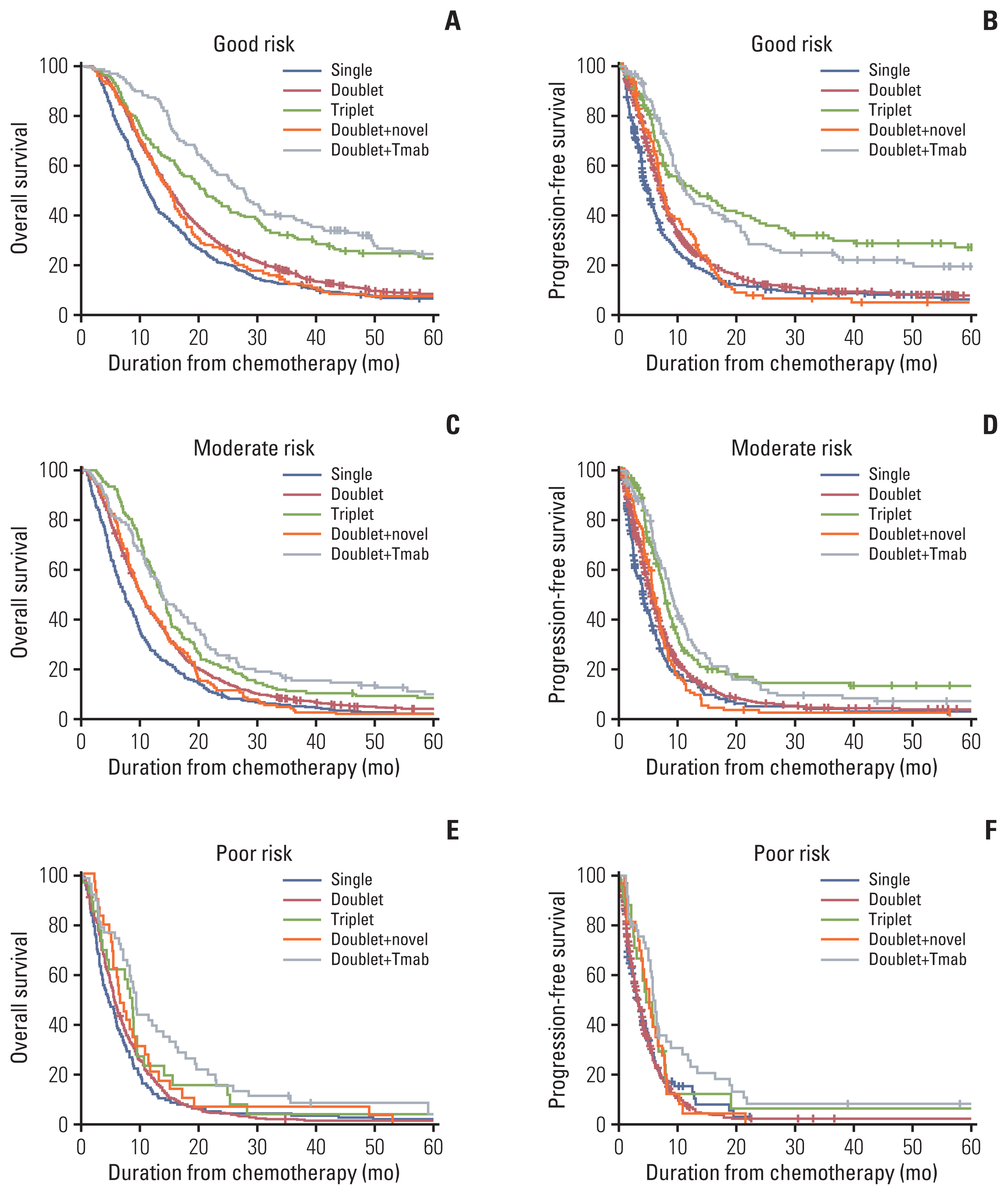
There were 880 patients (16%) in period 1, 1,573 (29%) in period 2, 1,435 (27%) in period 3, and 1,496 (28%) in period 4. Cytotoxic doublet-based therapy was the most commonly used (78%) first-line chemotherapy, and the combination of trastuzumab and doublet chemotherapy was provided to 288 patients. The OS rates at 12 and 24 months were steadily improved as follows: 39.2% and 14.6% in period 1, 43.5% and 17.6% in period 2, 50.3% and 20.6% in period 3, and 51.7% and 24.1% in period 4, respectively (p < 0.001). Among the patients who received the doublet-based chemotherapy, the median OS of those who received trastuzumab was 18.0 months (95% CI, 15.5–20.6), while that of those who received other doublet therapies was 11.2 months (95% CI, 10.8–11.6).
Conclusion
The OS was improved over time with advancements in chemotherapy, particularly the introduction of the anti-HER2–targeted agent, which contributed to the increase in the number of long-term survivors and established the superiority of OS for the treatment of MRGC.
Keyword
Figure
Cited by 1 articles
-
Varlitinib and Paclitaxel for EGFR/HER2 Co-expressing Advanced Gastric Cancer: A Multicenter Phase Ib/II Study (K-MASTER-13)
Dong-Hoe Koo, Minkyu Jung, Yeul Hong Kim, Hei-Cheul Jeung, Dae Young Zang, Woo Kyun Bae, Hyunki Kim, Hyo Song Kim, Choong-kun Lee, Woo Sun Kwon, Hyun Cheol Chung, Sun Young Rha
Cancer Res Treat. 2024;56(4):1136-1145. doi: 10.4143/crt.2023.1324.
Reference
-
References
1. Van Cutsem E, Sagaert X, Topal B, Haustermans K, Prenen H. Gastric cancer. Lancet. 2016; 388:2654–64.
Article2. Jung KW, Won YJ, Kong HJ, Lee ES. Prediction of cancer incidence and mortality in Korea, 2019. Cancer Res Treat. 2019; 51:431–7.
Article3. Guideline Committee of the Korean Gastric Cancer Association (KGCA), Development Working Group & Review Panel. Korean Practice Guideline for Gastric Cancer 2018: an evidence-based, multi-disciplinary approach. J Gastric Cancer. 2019; 19:1–48.4. Dekker E, Tanis PJ, Vleugels JL, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019; 394:1467–80.
Article5. Arbour KC, Riely GJ. Systemic therapy for locally advanced and metastatic non-small cell lung cancer: a review. JAMA. 2019; 322:764–74.6. Park YH, Lee JL, Ryoo BY, Ryu MH, Yang SH, Kim BS, et al. Capecitabine in combination with Oxaliplatin (XELOX) as a first-line therapy for advanced gastric cancer. Cancer Chemother Pharmacol. 2008; 61:623–9.
Article7. Kang YK, Kang WK, Shin DB, Chen J, Xiong J, Wang J, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009; 20:666–73.
Article8. Ryu MH, Baba E, Lee KH, Park YI, Boku N, Hyodo I, et al. Comparison of two different S-1 plus cisplatin dosing schedules as first-line chemotherapy for metastatic and/or recurrent gastric cancer: a multicenter, randomized phase III trial (SOS). Ann Oncol. 2015; 26:2097–101.
Article9. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010; 376:687–97.
Article10. Ohtsu A, Shah MA, Van Cutsem E, Rha SY, Sawaki A, Park SR, et al. Bevacizumab in combination with chemotherapy as first-line therapy in advanced gastric cancer: a randomized, double-blind, placebo-controlled phase III study. J Clin Oncol. 2011; 29:3968–76.
Article11. Lordick F, Kang YK, Chung HC, Salman P, Oh SC, Bodoky G, et al. Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol. 2013; 14:490–9.
Article12. Tabernero J, Hoff PM, Shen L, Ohtsu A, Shah MA, Cheng K, et al. Pertuzumab plus trastuzumab and chemotherapy for HER2-positive metastatic gastric or gastro-oesophageal junction cancer (JACOB): final analysis of a double-blind, randomised, placebo-controlled phase 3 study. Lancet Oncol. 2018; 19:1372–84.
Article13. Boku N, Ryu MH, Kato K, Chung HC, Minashi K, Lee KW, et al. Safety and efficacy of nivolumab in combination with S-1/capecitabine plus oxaliplatin in patients with previously untreated, unresectable, advanced, or recurrent gastric/gastroesophageal junction cancer: interim results of a randomized, phase II trial (ATTRACTION-4). Ann Oncol. 2019; 30:250–8.
Article14. Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014; 15:1224–35.
Article15. Fuchs CS, Tomasek J, Yong CJ, Dumitru F, Passalacqua R, Goswami C, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014; 383:31–9.
Article16. Chau I, Le DT, Ott PA, Korytowsky B, Le H, Le TK, et al. Developing real-world comparators for clinical trials in chemotherapy-refractory patients with gastric cancer or gastroesophageal junction cancer. Gastric Cancer. 2020; 23:133–41.
Article17. Koo DH, Ryu MH, Ryoo BY, Seo J, Lee MY, Chang HM, et al. Improving trends in survival of patients who receive chemotherapy for metastatic or recurrent gastric cancer: 12 years of experience at a single institution. Gastric Cancer. 2015; 18:346–53.
Article18. Koo DH, Ryoo BY, Kim HJ, Ryu MH, Lee SS, Moon JH, et al. A prognostic model in patients who receive chemotherapy for metastatic or recurrent gastric cancer: validation and comparison with previous models. Cancer Chemother Pharmacol. 2011; 68:913–21.
Article19. Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012; 30:1513–8.
Article20. Al-Batran SE, Homann N, Pauligk C, Goetze TO, Meiler J, Kasper S, et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): a randomised, phase 2/3 trial. Lancet. 2019; 393:1948–57.21. Kang YK, Yook JH, Park YK, Kim YW, Kim J, Ryu MH, et al. Phase III randomized study of neoadjuvant chemotherapy (CT) with docetaxel(D), oxaliplatin(O) and S-1(S) (DOS) followed by surgery and adjuvant S-1, vs surgery and adjuvant S-1, for resectable advanced gastric cancer (GC) (PRODIGY). Ann Oncol. 2019; 30(Suppl 5):v8767.
Article22. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol. 2017; 3:1237–44.23. Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer: a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer. 2011; 47:2306–14.24. Kang YK, Boku N, Satoh T, Ryu MH, Chao Y, Kato K, et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017; 390:2461–71.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Real-World Evidence of Trastuzumab, Pertuzumab, and Docetaxel Combination as a First-Line Treatment for Korean Patients with HER2-Positive Metastatic Breast Cancer
- Treatment for unresectable gastric cancer
- Prognostic Value of the Evolution of HER2-Low Expression after Neoadjuvant Chemotherapy
- Impact of Serum HER2 Levels on Survival and Its Correlation with Clinicopathological Parameters in Women with Breast Cancer
- Breakthroughs in the Systemic Treatment of HER2-Positive Advanced/Metastatic Gastric Cancer: From Singlet Chemotherapy to Triple Combination