Anat Cell Biol.
2021 Mar;54(1):74-82. 10.5115/acb.19.229.
Expression of neurotrophic factor genes by human adipose stem cells post-induction by deprenyl
- Affiliations
-
- 1Department of Cellular and Molecular Biology, School of Biology, Damghan University, Damghan, Iran
- KMID: 2514587
- DOI: http://doi.org/10.5115/acb.19.229
Abstract
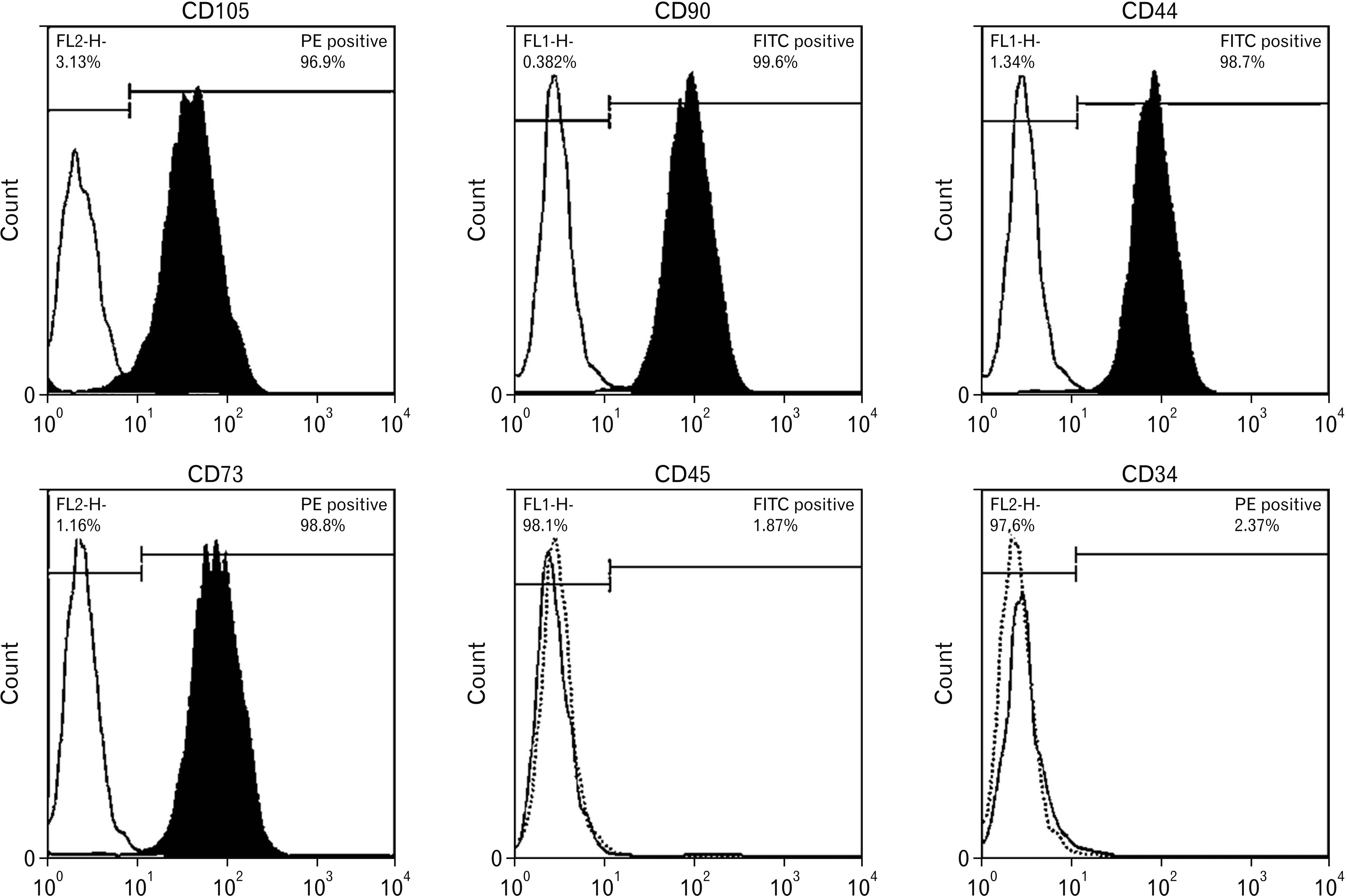
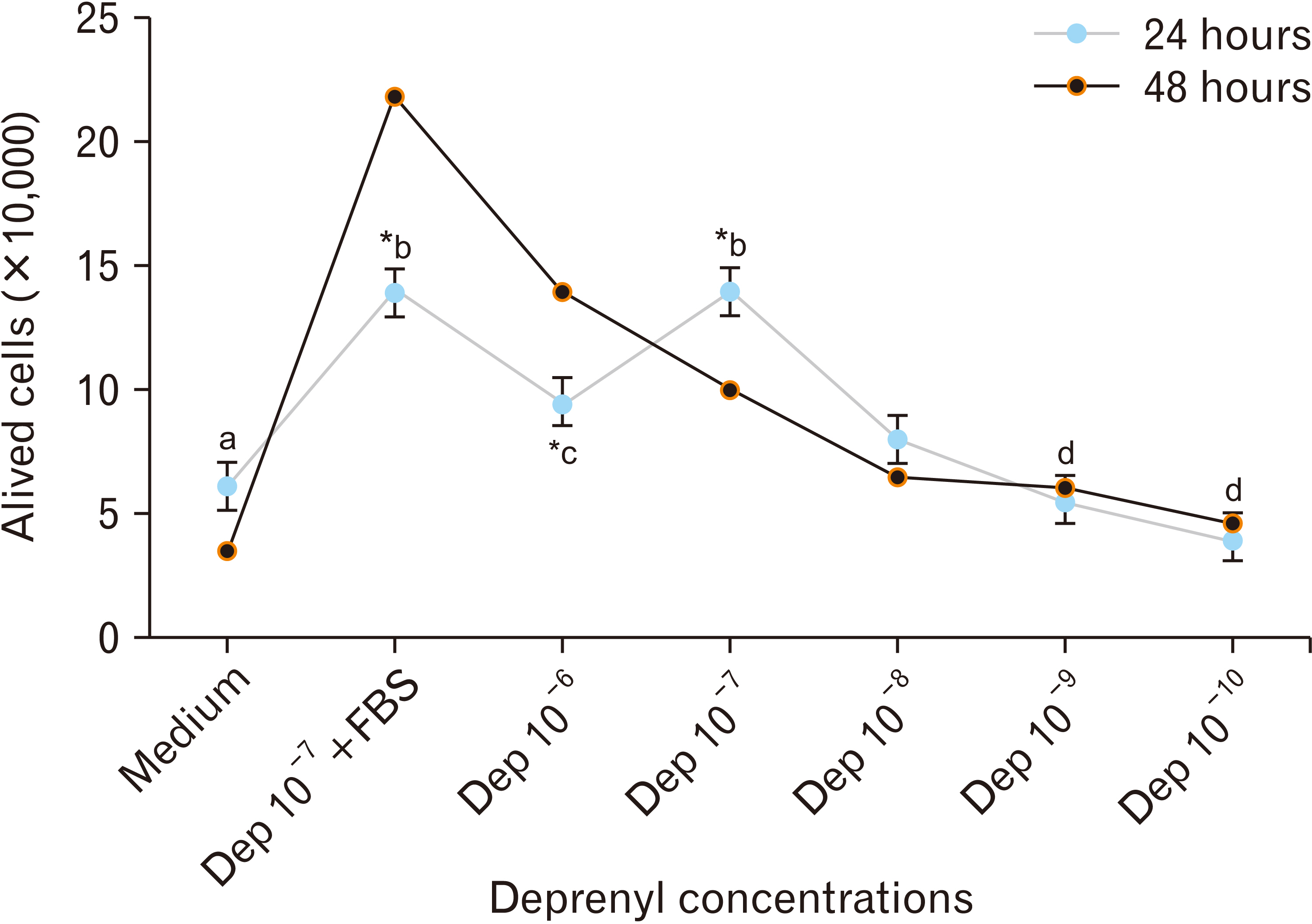
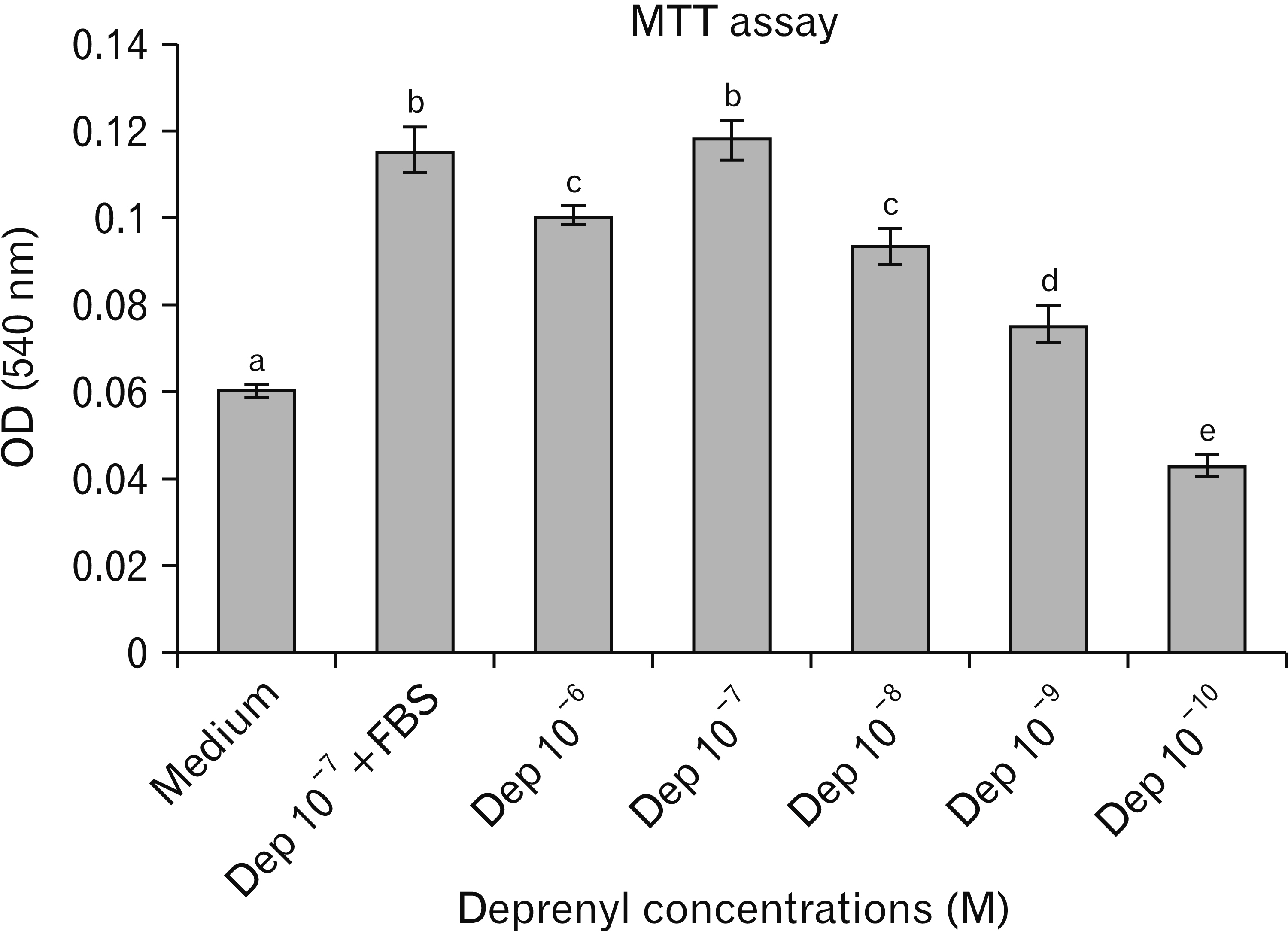
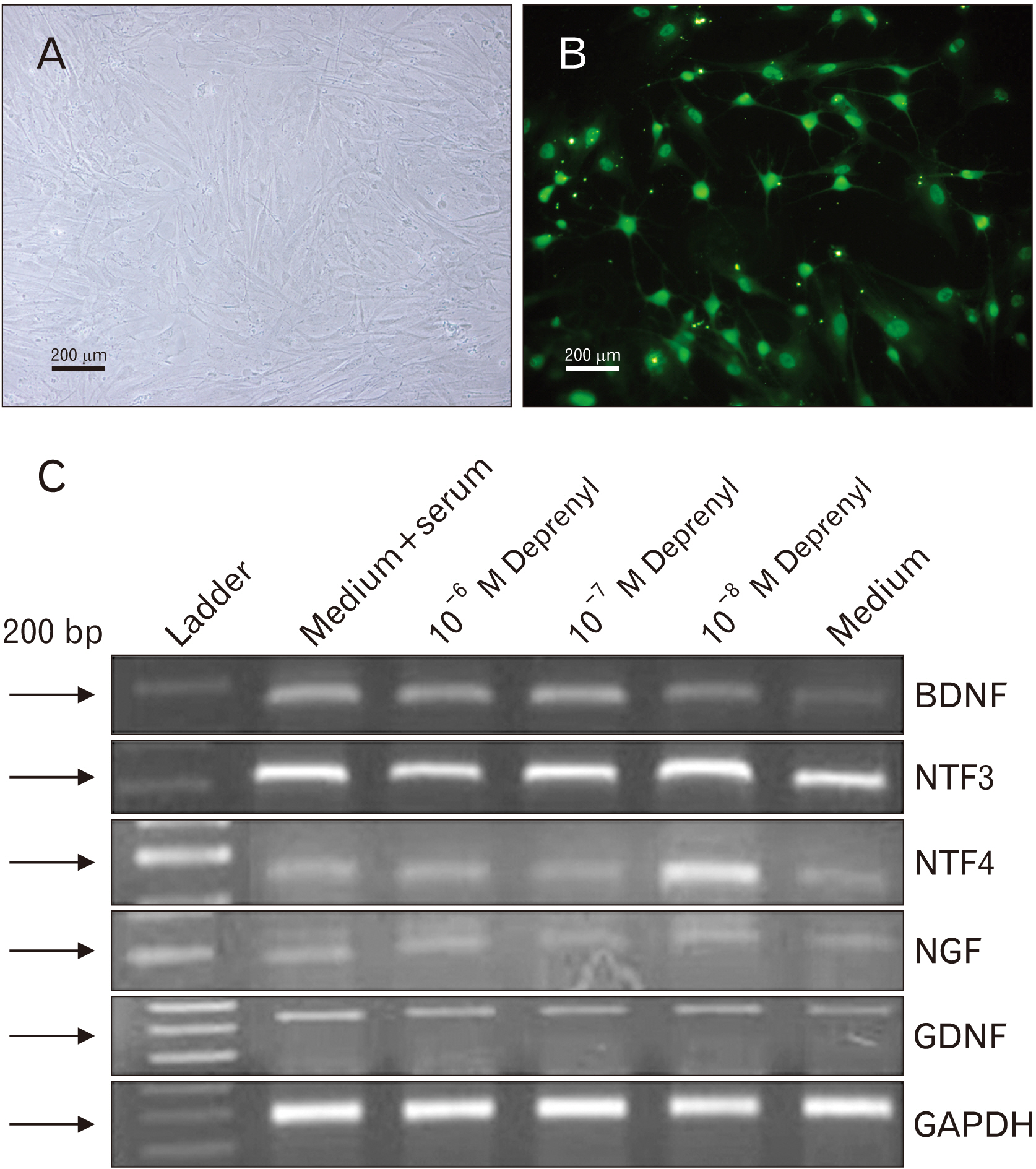
- Human adipose stem cells (hASCs) were introduced as appropriate candidate due to advantages like ease of isolation, in vitro expansion and lack of immune response. Deprenyl (Dep) was used to induce bone marrow stem cells into neuron-like cells. We investigated the Dep effect on neurotrophin genes expression in hASCs and their differentiation into neuron-like cells. The cells were isolated from small pieces of abdominal adipose tissue and subjected to flow cytometry to confirm purification. The osteogenic and adipogenic differentiation were identified. The proliferation rate and neurotrophin genes expression of treated cells were evaluated by MTT, TH immunostaining and RT-PCR. hASCs had positive response to CD44, CD73, CD90, CD105 markers and negative response to CD34 and CD45 markers and differentiated into adipocytes and osteocytes. Exposure to 10–7 M of Dep for 24 hours caused a significant increase of viable cells and BDNF, NTF-3 genes expression as compared to cultured cells in serum free medium and had no effect on the expression of NGF and GDNF genes. Based on our results, Dep is able to induce BDNF, NTF-3 and NTF-4 genes expression and neroun-like morphology in hASCs.
Figure
Reference
-
References
1. Ullah I, Subbarao RB, Rho GJ. 2015; Human mesenchymal stem cells- current trends and future prospective. Biosci Rep. 35:e00191. DOI: 10.1042/BSR20150025. PMID: 25797907. PMCID: PMC4413017.2. Volkman R, Offen D. 2017; Concise review: mesenchymal stem cells in neurodegenerative diseases. Stem Cells. 35:1867–80. DOI: 10.1002/stem.2651. PMID: 28589621.
Article3. Kariminekoo S, Movassaghpour A, Rahimzadeh A, Talebi M, Shamsasenjan K, Akbarzadeh A. 2016; Implications of mesenchymal stem cells in regenerative medicine. Artif Cells Nanomed Biotechnol. 44:749–57. DOI: 10.3109/21691401.2015.1129620. PMID: 26757594.
Article4. Frese L, Dijkman PE, Hoerstrup SP. 2016; Adipose tissue-derived stem cells in regenerative medicine. Transfus Med Hemother. 43:268–74. DOI: 10.1159/000448180. PMID: 27721702. PMCID: PMC5040903.
Article5. De Francesco F, Ricci G, D'Andrea F, Nicoletti GF, Ferraro GA. 2015; Human adipose stem cells: from bench to bedside. Tissue Eng Part B Rev. 21:572–84. DOI: 10.1089/ten.teb.2014.0608. PMID: 25953464.
Article6. Baer PC. 2014; Adipose-derived mesenchymal stromal/stem cells: an update on their phenotype in vivo and in vitro. World J Stem Cells. 6:256–65. DOI: 10.4252/wjsc.v6.i3.256. PMID: 25126376. PMCID: PMC4131268.7. Lindroos B, Suuronen R, Miettinen S. 2011; The potential of adipose stem cells in regenerative medicine. Stem Cell Rev Rep. 7:269–91. DOI: 10.1007/s12015-010-9193-7. PMID: 20853072.
Article8. Tabatabaei Qomi R, Sheykhhasan M. 2017; Adipose-derived stromal cell in regenerative medicine: a review. World J Stem Cells. 9:107–17. DOI: 10.4252/wjsc.v9.i8.107. PMID: 28928907. PMCID: PMC5583529.9. Burrow KL, Hoyland JA, Richardson SM. 2017; Human adipose-derived stem cells exhibit enhanced proliferative capacity and retain multipotency longer than donor-matched bone marrow mesenchymal stem cells during expansion in vitro. Stem Cells Int. 2017:2541275. DOI: 10.1155/2017/2541275. PMID: 28553357. PMCID: PMC5434475.10. Ciuffi S, Zonefrati R, Brandi ML. 2017; Adipose stem cells for bone tissue repair. Clin Cases Miner Bone Metab. 14:217–26. DOI: 10.11138/ccmbm/2017.14.1.217. PMID: 29263737. PMCID: PMC5726213.
Article11. Morizono K, De Ugarte DA, Zhu M, Zuk P, Elbarbary A, Ashjian P, Benhaim P, Chen IS, Hedrick MH. 2003; Multilineage cells from adipose tissue as gene delivery vehicles. Hum Gene Ther. 14:59–66. DOI: 10.1089/10430340360464714. PMID: 12573059.
Article12. Han C, Zhang L, Song L, Liu Y, Zou W, Piao H, Liu J. 2014; Human adipose-derived mesenchymal stem cells: a better cell source for nervous system regeneration. Chin Med J (Engl). 127:329–37. PMID: 24438624.13. Ebadi M, Sharma S, Shavali S, El Refaey H. 2002; Neuroprotective actions of selegiline. J Neurosci Res. 67:285–9. DOI: 10.1002/jnr.10148. PMID: 11813232.
Article14. Pálfi M, Szökó E, Kálmán M. 2006; [Molecular mechanisms of the neuroprotective effect of (-)-deprenyl]. Orv Hetil. 147:1251–7. Hungarian. PMID: 16927880.15. Magyar K. 2011; The pharmacology of selegiline. Int Rev Neurobiol. 100:65–84. DOI: 10.1016/B978-0-12-386467-3.00004-2. PMID: 21971003.
Article16. Knoll J. 2001; Antiaging compounds: (-)deprenyl (selegeline) and (-)1-(benzofuran-2-yl)-2-propylaminopentane, [(-)BPAP], a selective highly potent enhancer of the impulse propagation mediated release of catecholamine and serotonin in the brain. CNS Drug Rev. 7:317–45. DOI: 10.1111/j.1527-3458.2001.tb00202.x. PMID: 11607046. PMCID: PMC6494119.17. Naoi M, Maruyama W, Inaba-Hasegawa K. 2013; Revelation in the neuroprotective functions of rasagiline and selegiline: the induction of distinct genes by different mechanisms. Expert Rev Neurother. 13:671–84. DOI: 10.1586/ern.13.60. PMID: 23739004.
Article18. Magyar K, Szende B. 2004; (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology. 25:233–42. DOI: 10.1016/S0161-813X(03)00102-5. PMID: 14697898.
Article19. Miklya I. 2016; The significance of selegiline/(-)-deprenyl after 50 years in research and therapy (1965-2015). Mol Psychiatry. 21:1499–503. DOI: 10.1038/mp.2016.127. PMID: 27480491.
Article20. Kapoor VK, Dureja J, Chadha R. 2009; Synthetic drugs with anti-ageing effects. Drug Discov Today. 14:899–904. DOI: 10.1016/j.drudis.2009.07.006. PMID: 19638318.
Article21. Mitre M, Mariga A, Chao MV. 2017; Neurotrophin signalling: novel insights into mechanisms and pathophysiology. Clin Sci (Lond). 131:13–23. DOI: 10.1042/CS20160044. PMID: 27908981. PMCID: PMC5295469.
Article22. McAllister AK. 2001; Neurotrophins and neuronal differentiation in the central nervous system. Cell Mol Life Sci. 58:1054–60. DOI: 10.1007/PL00000920. PMID: 11529498.
Article23. Reichardt LF. 2006; Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 361:1545–64. DOI: 10.1098/rstb.2006.1894. PMID: 16939974. PMCID: PMC1664664.
Article24. Deister C, Schmidt CE. 2006; Optimizing neurotrophic factor combinations for neurite outgrowth. J Neural Eng. 3:172–9. DOI: 10.1088/1741-2560/3/2/011. PMID: 16705273.
Article25. Schmidt N, Schulze J, Warwas DP, Ehlert N, Lenarz T, Warnecke A, Behrens P. 2018; Long-term delivery of brain-derived neurotrophic factor (BDNF) from nanoporous silica nanoparticles improves the survival of spiral ganglion neurons in vitro. PLoS One. 13:e0194778. DOI: 10.1371/journal.pone.0194778. PMID: 29584754. PMCID: PMC5870973.26. Lindsay RM, Alderson RF, Friedman B, Hyman C, Ip NY, Furth ME, Maisonpierre PC, Squinto SP, Yancopoulos GD. 1991; The neurotrophin family of NGF-related neurotrophic factors. Restor Neurol Neurosci. 2:211–20. DOI: 10.3233/RNN-1991-245608. PMID: 21551605.
Article27. Keefe KM, Sheikh IS, Smith GM. 2017; Targeting neurotrophins to specific populations of neurons: NGF, BDNF, and NT-3 and their relevance for treatment of spinal cord injury. Int J Mol Sci. 18:548. DOI: 10.3390/ijms18030548. PMID: 28273811. PMCID: PMC5372564.
Article28. Snider WD, Wright DE. 1996; Neurotrophins cause a new sensation. Neuron. 16:229–32. DOI: 10.1016/S0896-6273(00)80039-2. PMID: 8789936.
Article29. Henderson CE. 1996; Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol. 6:64–70. DOI: 10.1016/S0959-4388(96)80010-9. PMID: 8794045.
Article30. Ernsberger U. 2008; The role of GDNF family ligand signalling in the differentiation of sympathetic and dorsal root ganglion neurons. Cell Tissue Res. 333:353–71. DOI: 10.1007/s00441-008-0634-4. PMID: 18629541. PMCID: PMC2516536.
Article31. Esmaeili F, Tiraihi T, Movahedin M, Mowla SJ. 2006; Selegiline induces neuronal phenotype and neurotrophins expression in embryonic stem cells. Rejuvenation Res. 9:475–84. DOI: 10.1089/rej.2006.9.475. PMID: 17105388.
Article32. Ghorbanian MT, Tiraihi T, Mesbah-Namin SA, Fathollahi Y. 2010; Selegiline is an efficient and potent inducer for bone marrow stromal cell differentiation into neuronal phenotype. Neurol Res. 32:185–93. DOI: 10.1179/174313209X409016. PMID: 19422735.
Article33. Bakhshalizadeh S, Esmaeili F, Houshmand F, Shirzad H, Saedi M. 2011; Effects of selegiline, a monoamine oxidase B inhibitor, on differentiation of P19 embryonal carcinoma stem cells, into neuron-like cells. In Vitro Cell Dev Biol Anim. 47:550–7. DOI: 10.1007/s11626-011-9442-3. PMID: 21858609.
Article34. Mizuta I, Ohta M, Ohta K, Nishimura M, Mizuta E, Hayashi K, Kuno S. 2000; Selegiline and desmethylselegiline stimulate NGF, BDNF, and GDNF synthesis in cultured mouse astrocytes. Biochem Biophys Res Commun. 279:751–5. DOI: 10.1006/bbrc.2000.4037. PMID: 11162424.
Article35. Hassanzadeh K, Nikzaban M, Moloudi MR, Izadpanah E. 2015; Effect of selegiline on neural stem cells differentiation: a possible role for neurotrophic factors. Iran J Basic Med Sci. 18:549–54. PMID: 26221478. PMCID: PMC4509949.36. Kornicka K, Marycz K, Tomaszewski KA, Marędziak M, Śmieszek A. 2015; The effect of age on osteogenic and adipogenic differentiation potential of human adipose derived stromal stem cells (hASCs) and the impact of stress factors in the course of the differentiation process. Oxid Med Cell Longev. 2015:309169. DOI: 10.1155/2015/309169. PMID: 26246868. PMCID: PMC4515302.
Article37. Younesi E, Bayati V, Hashemitabar M, Azandeh SS, Bijannejad D, Bahreini A. 2015; Differentiation of adipose-derived stem cells into Schwann-like cells: fetal bovine serum or human serum? Anat Cell Biol. 48:170–6. DOI: 10.5115/acb.2015.48.3.170. PMID: 26417476. PMCID: PMC4582159.
Article38. Strioga M, Viswanathan S, Darinskas A, Slaby O, Michalek J. 2012; Same or not the same? Comparison of adipose tissue-derived versus bone marrow-derived mesenchymal stem and stromal cells. Stem Cells Dev. 21:2724–52. DOI: 10.1089/scd.2011.0722. PMID: 22468918.
Article39. Abdanipour A, Tiraihi T, Delshad A. 2011; Trans-differentiation of the adipose tissue-derived stem cells into neuron-like cells expressing neurotrophins by selegiline. Iran Biomed J. 15:113–21. DOI: 10.6091/ibj.1011.2012. PMID: 22395135. PMCID: PMC3614245.40. Maruyama W, Naoi M. 2013; "70th Birthday Professor Riederer" induction of glial cell line-derived and brain-derived neurotrophic factors by rasagiline and (-)deprenyl: a way to a disease-modifying therapy? J Neural Transm (Vienna). 120:83–9. DOI: 10.1007/s00702-012-0876-x. PMID: 22892822.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Expression of mRNAs for Neurotrophic Factors in Human Neural Stem Cells Derived from Fetal Telencephalon
- In Vitro Study of Adipose-Derived Mesenchymal Stem Cells Transduced with Lentiviral Vector Carrying the Brain-Derived Neurotrophic Factor Gene
- Cardiomyogenic Potential of Human Adipose Tissue and Umbilical Cord Derived-Mesenchymal Like Stem Cells
- The Effect of Recombinant Tyrosine Hydroxylase Expression on the Neurogenic Differentiation Potency of Mesenchymal Stem Cells
- Adipose Stem Cells as Alternatives for Bone Marrow Mesenchymal Stem Cells in Oral Ulcer Healing