Clin Endosc.
2021 Mar;54(2):261-268. 10.5946/ce.2020.101.
Endoscopic Ultrasound-Guided Fine Needle Biopsy Needles Provide Higher Diagnostic Yield Compared to Endoscopic Ultrasound-Guided Fine Needle Aspiration Needles When Sampling Solid Pancreatic Lesions: A Meta-Analysis
- Affiliations
-
- 1Division of Gastroenterology and Hepatobiliary Disease, New York-Presbyterian Brooklyn Methodist Hospital, Brooklyn, NY, USA
- 2Department of Internal Medicine, Wright State University, Dayton, OH, USA
- 3Department of Epidemiology and Biostatistics, Weill Cornell Medical College, New York, NY, USA
- 4Division of Gastroenterology and Hepatology, New York Presbyterian, Columbia University Medical Center, New York, NY, USA
- KMID: 2514182
- DOI: http://doi.org/10.5946/ce.2020.101
Abstract
- Background/Aims
Studies comparing the utility of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) and endoscopic ultrasound-guided fine needle biopsy (EUS-FNB) for solid pancreatic lesions have been inconclusive with no clear superiority. The aim of this meta-analysis was to compare the diagnostic accuracy and safety between the two sampling techniques.
Methods
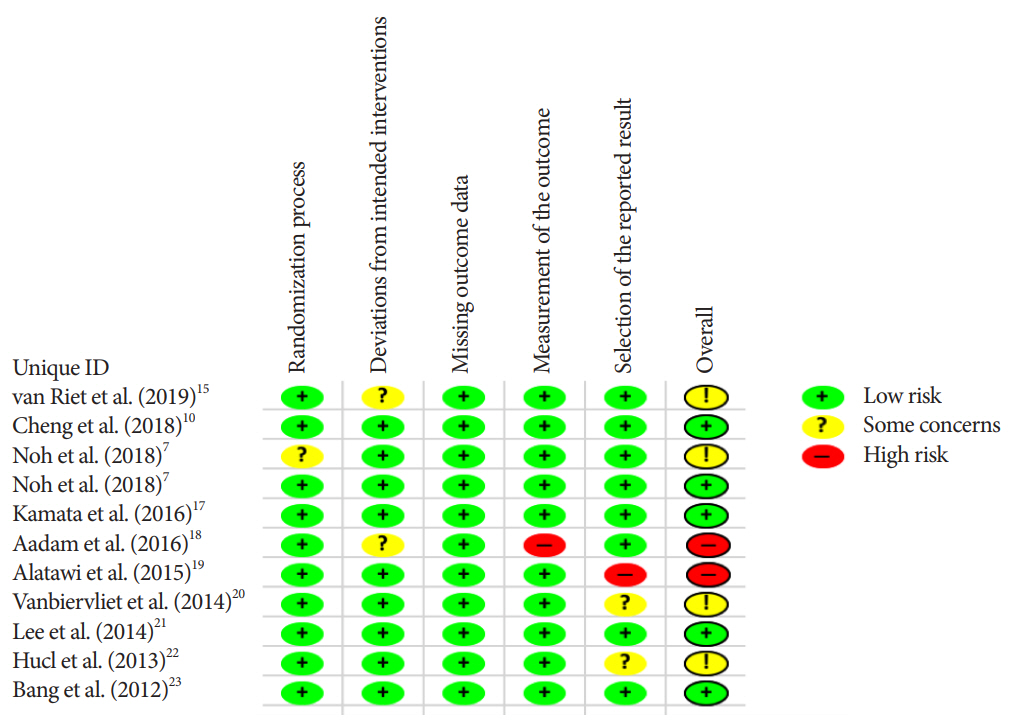
We performed a systematic search of randomized controlled trials published between 2012 and 2019. The primary outcome was overall diagnostic accuracy. Secondary outcomes included adverse event rates, cytopathologic and histopathologic accuracy, and the mean number of passes required to obtain adequate tissue between FNA and FNB needles. Fixed and random effect models with pooled estimates of target outcomes were developed.
Results
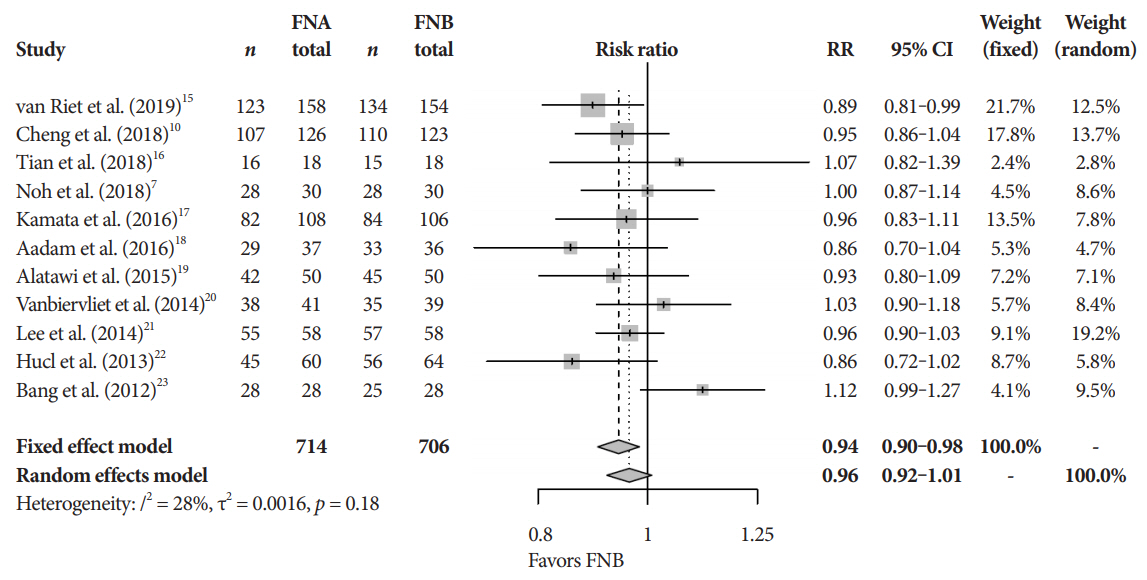
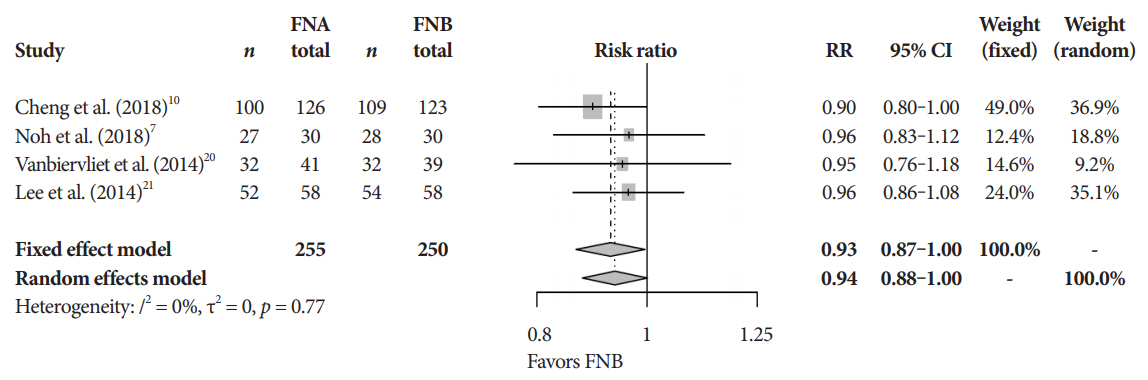
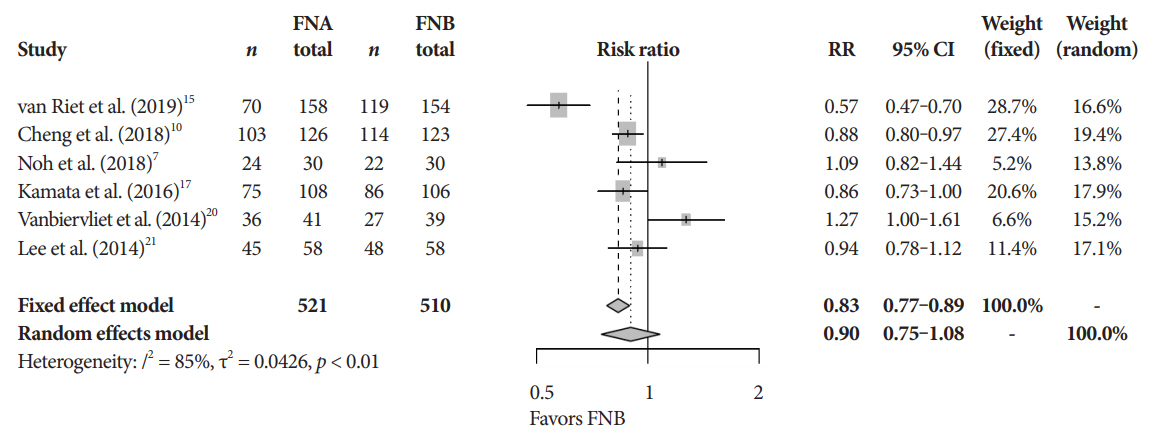
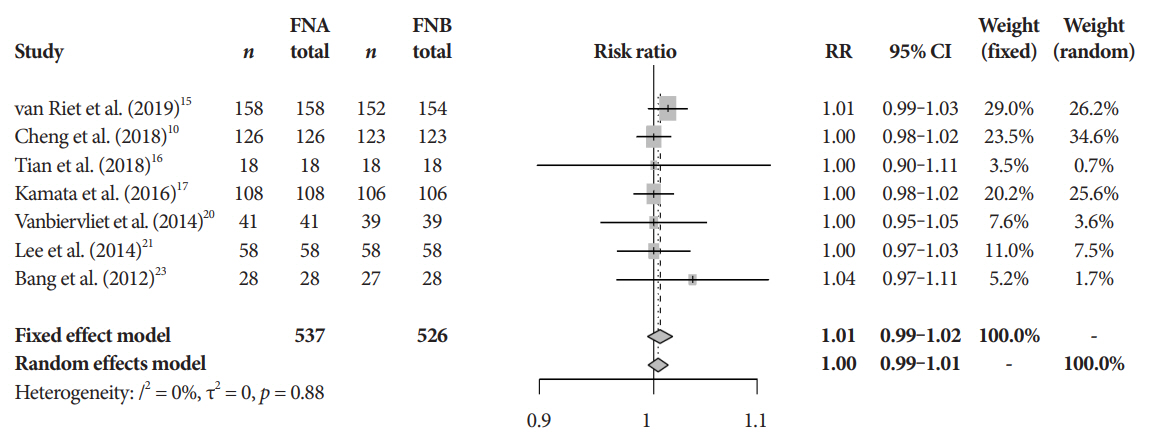
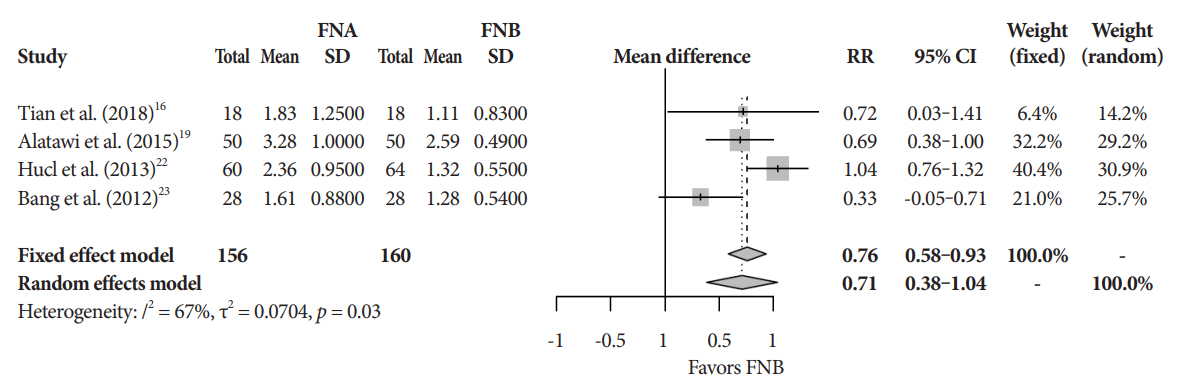
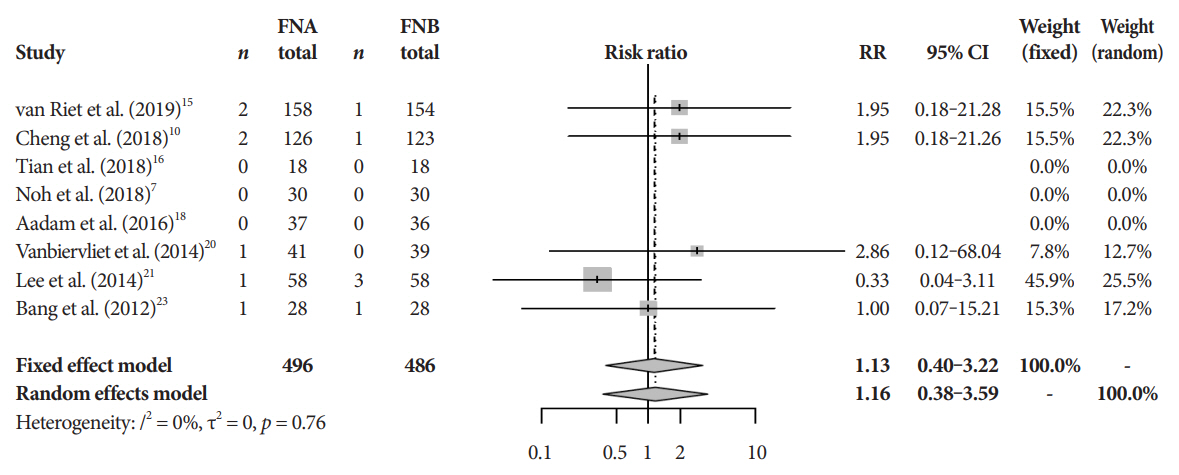
Eleven studies involving 1,365 participants were included for analysis. When compared to FNB, FNA had a significant reduction in diagnostic accuracy (81% and 87%, p=0.005). In addition, FNA provided reduced cytopathologic accuracy (82% and 89%, p=0.04) and an increased number of mean passes required compared to FNB (2.3 and 1.6, respectively, p<0.0001). There was no difference in adverse event rate between FNA and FNB needles (1.8% and 2.3% respectively, p=0.64).
Conclusions
FNB provides superior diagnostic accuracy without compromising safety when compared to FNA. FNB should be readily considered by endosonographers when evaluating solid pancreatic masses.
Figure
Reference
-
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018; 68:7–30.
Article2. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69:7–34.
Article3. Kleeff J, Korc M, Apte M, et al. Pancreatic cancer. Nat Rev Dis Primers. 2016; 2:16022.
Article4. Nayar MK, Paranandi B, Dawwas MF, et al. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017; 85:1017–1024.
Article5. Ishikawa T, Mohamed R, Heitman SJ, et al. Diagnostic yield of small histological cores obtained with a new EUS-guided fine needle biopsy system. Surg Endosc. 2017; 31:5143–5149.
Article6. Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012; 75:319–331.
Article7. Noh DH, Choi K, Gu S, et al. Comparison of 22-gauge standard fine needle versus core biopsy needle for endoscopic ultrasound-guided sampling of suspected pancreatic cancer: a randomized crossover trial. Scand J Gastroenterol. 2018; 53:94–99.
Article8. Nagula S, Pourmand K, Aslanian H, et al. Comparison of endoscopic ultrasound-fine-needle aspiration and endoscopic ultrasound-fine-needle biopsy for solid lesions in a multicenter, randomized trial. Clin Gastroenterol Hepatol. 2018; 16:1307–1313.e1.9. Hedenström P, Demir A, Khodakaram K, Nilsson O, Sadik R. EUS-guided reverse bevel fine-needle biopsy sampling and open tip fine-needle aspiration in solid pancreatic lesions - a prospective, comparative study. Scand J Gastroenterol. 2018; 53:231–237.
Article10. Cheng B, Zhang Y, Chen Q, et al. Analysis of fine-needle biopsy vs fine-needle aspiration in diagnosis of pancreatic and abdominal masses: a prospective, multicenter, randomized controlled trial. Clin Gastroenterol Hepatol. 2018; 16:1314–1321.
Article11. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009; 151:W65–W94.
Article12. Higgins JP, Altman DG, Gøtzsche PC, et al. The Cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011; 343:d5928.
Article13. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.
Article14. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002; 21:1539–1558.
Article15. van Riet PA, Larghi A, Attili F, et al. A multicenter randomized trial comparing a 25-gauge EUS fine-needle aspiration device with a 20-gauge EUS fine-needle biopsy device. Gastrointest Endosc. 2019; 89:329–339.
Article16. Tian L, Tang AL, Zhang L, et al. Evaluation of 22G fine-needle aspiration (FNA) versus fine-needle biopsy (FNB) for endoscopic ultrasound-guided sampling of pancreatic lesions: a prospective comparison study. Surg Endosc. 2018; 32:3533–3539.
Article17. Kamata K, Kitano M, Yasukawa S, et al. Histologic diagnosis of pancreatic masses using 25-gauge endoscopic ultrasound needles with and without a core trap: a multicenter randomized trial. Endoscopy. 2016; 48:632–638.
Article18. Aadam AA, Wani S, Amick A, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016; 4:E497–E505.
Article19. Alatawi A, Beuvon F, Grabar S, et al. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015; 3:343–352.
Article20. Vanbiervliet G, Napoléon B, Saint Paul MC, et al. Core needle versus standard needle for endoscopic ultrasound-guided biopsy of solid pancreatic masses: a randomized crossover study. Endoscopy. 2014; 46:1063–1070.
Article21. Lee YN, Moon JH, Kim HK, et al. Core biopsy needle versus standard aspiration needle for endoscopic ultrasound-guided sampling of solid pancreatic masses: a randomized parallel-group study. Endoscopy. 2014; 46:1056–1062.
Article22. Hucl T, Wee E, Anuradha S, et al. Feasibility and efficiency of a new 22G core needle: a prospective comparison study. Endoscopy. 2013; 45:792–798.
Article23. Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012; 76:321–327.
Article24. Li H, Li W, Zhou QY, Fan B. Fine needle biopsy is superior to fine needle aspiration in endoscopic ultrasound guided sampling of pancreatic masses: a meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018; 97:e0207.25. Wang J, Zhao S, Chen Y, Jia R, Zhang X. Endoscopic ultrasound guided fine needle aspiration versus endoscopic ultrasound guided fine needle biopsy in sampling pancreatic masses: a meta-analysis. Medicine (Baltimore). 2017; 96:e7452.26. Conti CB, Cereatti F, Grassia R. Endoscopic ultrasound-guided sampling of solid pancreatic masses: the fine needle aspiration or fine needle biopsy dilemma. Is the best needle yet to come? World J Gastrointest Endosc. 2019; 11:454–471.
Article27. Mohan BP, Shakhatreh M, Garg R, Ponnada S, Adler DG. Efficacy and safety of EUS-guided liver biopsy: a systematic review and meta-analysis. Gastrointest Endosc. 2019; 89:238–246.e3.
Article28. Okasha HH, Naga MI, Esmat S, et al. Endoscopic ultrasound-guided fine needle aspiration versus percutaneous ultrasound-guided fine needle aspiration in diagnosis of focal pancreatic masses. Endosc Ultrasound. 2013; 2:190–193.
Article29. Mohamadnejad M, Mullady D, Early DS, et al. Increasing number of passes beyond 4 does not increase sensitivity of detection of pancreatic malignancy by endoscopic ultrasound-guided fine-needle aspiration. Clin Gastroenterol Hepatol. 2017; 15:1071–1078.e2.
Article30. Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000; 51:184–190.
Article31. Abdelfatah MM, Grimm IS, Gangarosa LM, Baron TH. Cohort study comparing the diagnostic yields of 2 different EUS fine-needle biopsy needles. Gastrointest Endosc. 2018; 87:495–500.
Article32. Facciorusso A, Del Prete V, Buccino VR, Purohit P, Setia P, Muscatiello N. Diagnostic yield of Franseen and fork-tip biopsy needles for endoscopic ultrasound-guided tissue acquisition: a meta-analysis. Endosc Int Open. 2019; 7:E1221–E1230.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- How to optimize the diagnostic yield of endoscopic ultrasoundguided fine-needle sampling in solid pancreatic lesions from a technical perspective
- Endoscopic Ultrasound-Fine Needle Aspiration versus Core Biopsy for the Diagnosis of Subepithelial Tumors
- Fine-Needle Biopsy: Should This Be the First Choice in Endoscopic Ultrasound-Guided Tissue Acquisition?
- How Can We Get the Best Results with Endoscopic Ultrasound-Guided Fine Needle Aspiration?
- Endoscopic Ultrasound-Guided Direct Intervention for Solid Pancreatic Tumors