J Korean Med Sci.
2021 Mar;36(12):e80. 10.3346/jkms.2021.36.e80.
Effects of Wearable Powered Exoskeletal Training on Functional Mobility, Physiological Health and Quality of Life in Non-ambulatory Spinal Cord Injury Patients
- Affiliations
-
- 1Department of Rehabilitation Medicine, Hanyang University College of Medicine, Seoul, Korea
- 2Department of Rehabilitation Medicine, Hanyang University Guri Hospital, Guri, Korea
- 3Robotics Lab., R&D Division of Hyundai Motor Company, Uiwang, Korea
- 4Department of Nuclear Medicine, Hanyang University College of Medicine, Seoul, Korea
- KMID: 2514125
- DOI: http://doi.org/10.3346/jkms.2021.36.e80
Abstract
- Background
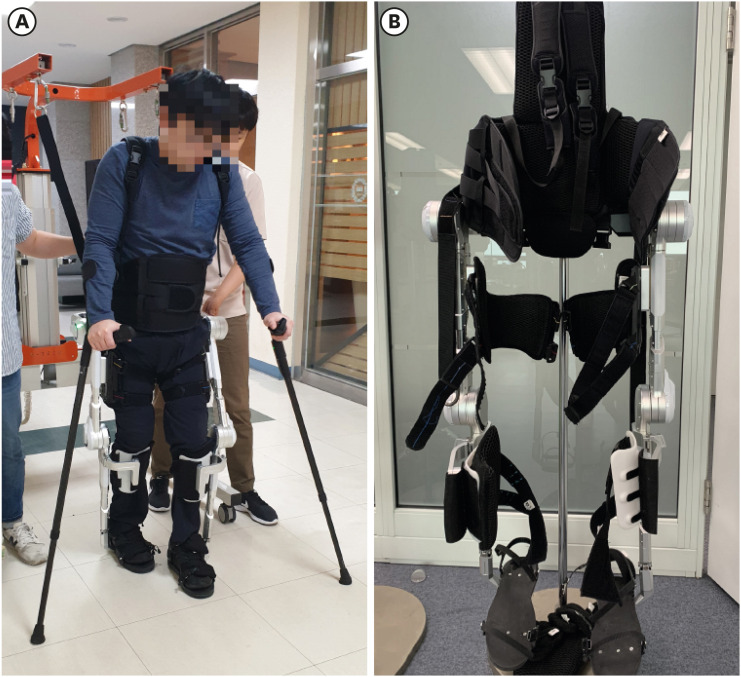
Spinal cord injury (SCI) is a serious clinical condition that impacts a patient's physical, psychological, and socio-economic status. The aim of this pilot study was to evaluate the effects of training with a newly developed powered wearable exoskeleton (Hyundai Medical Exoskeleton [H-MEX]) on functional mobility, physiological health, and quality of life in non-ambulatory SCI patients.
Methods
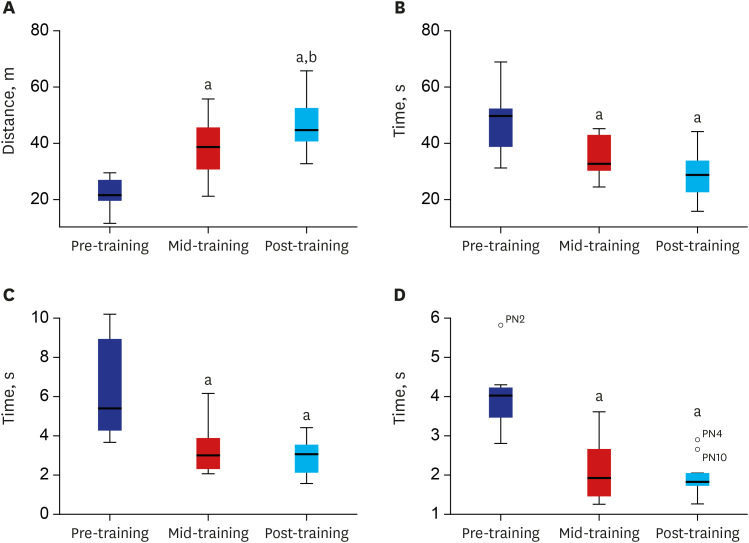
Participants received 60 minutes of walking training with a powered exoskeleton 3 times per week for 10 weeks (total 30 sessions). The 6-minute walking test (6MWT) and timedup-and-go test (TUGT) were performed to assess ambulatory function. The physiological outcomes of interest after exoskeleton-assisted walking training were spasticity, pulmonary function, bone mineral density, colon transit time, and serum inflammatory markers. Effects of walking training on subjective outcomes were estimated by the Korean version of the Falls Efficacy Scale—International and the 36-Item Short-Form Health Survey version 2.
Results
Ten participants finished 30 sessions of training and could ambulate independently.No severe adverse events were reported during the study. After training, the mean distance walked in the 6MWT (49.13 m) was significantly enhanced compared with baseline (20.65 m). The results of the TUGT also indicated a statistically significant improvement in the times required to stand up, walk 3 m and sit down. Although not statistically significant, clinically meaningful changes in some secondary physiological outcomes and/or quality of life were reported in some participants.
Conclusion
In conclusion, this study demonstrated that the newly developed wearable exoskeleton, H-MEX is safe and feasible for non-ambulatory SCI patients, and may have potential to improve quality of life of patients by assisting bipedal ambulation. These results suggest that the H-MEX can be considered a beneficial device for chronic non-ambulatory SCI patients.
Figure
Reference
-
1. Craig A, Tran Y, Middleton J. Psychological morbidity and spinal cord injury: a systematic review. Spinal Cord. 2009; 47(2):108–114. PMID: 18779835.
Article2. Priebe MM, Chiodo AE, Scelza WM, Kirshblum SC, Wuermser LA, Ho CH. Spinal cord injury medicine. 6. Economic and societal issues in spinal cord injury. Arch Phys Med Rehabil. 2007; 88(3):Suppl 1. S84–S88. PMID: 17321854.
Article3. Choi SH, Sung CH, Heo DR, Jeong SY, Kang CN. Incidence of acute spinal cord injury and associated complications of methylprednisolone therapy: a national population-based study in South Korea. Spinal Cord. 2020; 58(2):232–237. PMID: 31527724.
Article4. Singh A, Tetreault L, Kalsi-Ryan S, Nouri A, Fehlings MG. Global prevalence and incidence of traumatic spinal cord injury. Clin Epidemiol. 2014; 6:309–331. PMID: 25278785.5. Sezer N, Akkuş S, Uğurlu FG. Chronic complications of spinal cord injury. World J Orthop. 2015; 6(1):24–33. PMID: 25621208.
Article6. Piepmeier JM, Jenkins NR. Late neurological changes following traumatic spinal cord injury. J Neurosurg. 1988; 69(3):399–402. PMID: 3404238.
Article7. Bergmann P, Heilporn A, Schoutens A, Paternot J, Tricot A. Longitudinal study of calcium and bone metabolism in paraplegic patients. Paraplegia. 1977; 15(2):147–159. PMID: 198726.
Article8. Lazo MG, Shirazi P, Sam M, Giobbie-Hurder A, Blacconiere MJ, Muppidi M. Osteoporosis and risk of fracture in men with spinal cord injury. Spinal Cord. 2001; 39(4):208–214. PMID: 11420736.
Article9. Morse LR, Stolzmann K, Nguyen HP, Jain NB, Zayac C, Gagnon DR, et al. Association between mobility mode and C-reactive protein levels in men with chronic spinal cord injury. Arch Phys Med Rehabil. 2008; 89(4):726–731. PMID: 18374004.
Article10. Hurley EA. Use of KAFOs for patients with cerebral vascular accident, traumatic brain injury, and spinal cord injury. J Prosthet Orthot. 2006; 18(7):P199–201.
Article11. Waters RL, Lunsford BR. Energy cost of paraplegic locomotion. J Bone Joint Surg Am. 1985; 67(8):1245–1250. PMID: 4055849.
Article12. Spungen AM, Asselin PK, Fineberg DB, Kornfeld SD, Harel NY. Exoskeletal-assisted walking for persons with motor-complete paraplegia. Updated 2013. Accessed Septmeber 29, 2020. http://www.ryzur.com.cn/uploadfile/2016/0830/20160830115519272.pdf.13. Kozlowski AJ, Bryce TN, Dijkers MP. Time and effort required by persons with spinal cord injury to learn to use a powered exoskeleton for assisted walking. Top Spinal Cord Inj Rehabil. 2015; 21(2):110–121. PMID: 26364280.
Article14. Aach M, Cruciger O, Sczesny-Kaiser M, Höffken O, Meindl RC, Tegenthoff M, et al. Voluntary driven exoskeleton as a new tool for rehabilitation in chronic spinal cord injury: a pilot study. Spine J. 2014; 14(12):2847–2853. PMID: 24704677.15. Hartigan C, Kandilakis C, Dalley S, Clausen M, Wilson E, Morrison S, et al. Mobility outcomes following five training sessions with a powered exoskeleton. Top Spinal Cord Inj Rehabil. 2015; 21(2):93–99. PMID: 26364278.
Article16. Lee HD, Han CS. Technical trend of the lower limb exoskeleton system for the performance enhancement. J Inst Contr Robot Syst. 2014; 20(3):364–371.
Article17. Esquenazi A, Talaty M, Packel A, Saulino M. The ReWalk powered exoskeleton to restore ambulatory function to individuals with thoracic-level motor-complete spinal cord injury. Am J Phys Med Rehabil. 2012; 91(11):911–921. PMID: 23085703.
Article18. Zeilig G, Weingarden H, Zwecker M, Dudkiewicz I, Bloch A, Esquenazi A. Safety and tolerance of the ReWalk™ exoskeleton suit for ambulation by people with complete spinal cord injury: a pilot study. J Spinal Cord Med. 2012; 35(2):96–101. PMID: 22333043.19. Kolakowsky-Hayner SA, Crew J, Moran S, Shah A. Safety and feasibility of using the EksoTM bionic exoskeleton to aid ambulation after spinal cord injury. J Spine. 2013; S4:003.
Article20. Sczesny-Kaiser M, Höffken O, Aach M, Cruciger O, Grasmücke D, Meindl R, et al. HAL® exoskeleton training improves walking parameters and normalizes cortical excitability in primary somatosensory cortex in spinal cord injury patients. J Neuroeng Rehabil. 2015; 12(1):68. PMID: 26289818.
Article21. Benson I, Hart K, Tussler D, van Middendorp JJ. Lower-limb exoskeletons for individuals with chronic spinal cord injury: findings from a feasibility study. Clin Rehabil. 2016; 30(1):73–84. PMID: 25761635.
Article22. Miller LE, Zimmermann AK, Herbert WG. Clinical effectiveness and safety of powered exoskeleton-assisted walking in patients with spinal cord injury: systematic review with meta-analysis. Med Devices (Auckl). 2016; 9:455–466. PMID: 27042146.
Article23. Delgado AD, Escalon MX, Bryce TN, Weinrauch W, Suarez SJ, Kozlowski AJ. Safety and feasibility of exoskeleton-assisted walking during acute/sub-acute SCI in an inpatient rehabilitation facility: a single-group preliminary study. J Spinal Cord Med. 2020; 43(5):657–666. PMID: 31603395.
Article24. Hyun DJ, Lim H, Park S, Yoon J, Jung K, Bae K, et al. Walking propulsion generation in double stance by powered exoskeleton for paraplegics. Robot Auton Syst. 2019; 116:24–37.
Article25. Wu CH, Mao HF, Hu JS, Wang TY, Tsai YJ, Hsu WL. The effects of gait training using powered lower limb exoskeleton robot on individuals with complete spinal cord injury. J Neuroeng Rehabil. 2018; 15(1):14. PMID: 29506530.
Article26. Kwon SH, Lee BS, Lee HJ, Kim EJ, Lee JA, Yang SP, et al. Energy efficiency and patient satisfaction of gait with knee-ankle-foot orthosis and robot (ReWalk)-assisted gait in patients with spinal cord injury. Ann Rehabil Med. 2020; 44(2):131–141. PMID: 32392652.
Article27. Stampacchia G, Rustici A, Bigazzi S, Gerini A, Tombini T, Mazzoleni S. Walking with a powered robotic exoskeleton: subjective experience, spasticity and pain in spinal cord injured persons. NeuroRehabilitation. 2016; 39(2):277–283. PMID: 27372363.
Article28. Khan AS, Livingstone DC, Hurd CL, Duchcherer J, Misiaszek JE, Gorassini MA, et al. Retraining walking over ground in a powered exoskeleton after spinal cord injury: a prospective cohort study to examine functional gains and neuroplasticity. J Neuroeng Rehabil. 2019; 16(1):145. PMID: 31752911.
Article29. Mirbagheri MM, Kindig MW, Niu X. Effects of robotic-locomotor training on stretch reflex function and muscular properties in individuals with spinal cord injury. Clin Neurophysiol. 2015; 126(5):997–1006. PMID: 25449559.
Article30. Soyupek F, Savas S, Oztürk O, Ilgün E, Bircan A, Akkaya A. Effects of body weight supported treadmill training on cardiac and pulmonary functions in the patients with incomplete spinal cord injury. J Back Musculoskeletal Rehabil. 2009; 22(4):213–218.
Article31. Akkurt H, Karapolat HU, Kirazli Y, Kose T. The effects of upper extremity aerobic exercise in patients with spinal cord injury: a randomized controlled study. Eur J Phys Rehabil Med. 2017; 53(2):219–227. PMID: 27824234.32. Ries AL, Bauldoff GS, Carlin BW, Casaburi R, Emery CF, Mahler DA, et al. Pulmonary rehabilitation: joint ACCP/AACVPR evidence-based clinical practice guidelines. Chest. 2007; 131(5):Suppl. 4S–42S. PMID: 17494825.33. Zehnder Y, Lüthi M, Michel D, Knecht H, Perrelet R, Neto I, et al. Long-term changes in bone metabolism, bone mineral density, quantitative ultrasound parameters, and fracture incidence after spinal cord injury: a cross-sectional observational study in 100 paraplegic men. Osteoporos Int. 2004; 15(3):180–189. PMID: 14722626.
Article34. Karelis AD, Carvalho LP, Castillo MJ, Gagnon DH, Aubertin-Leheudre M. Effect on body composition and bone mineral density of walking with a robotic exoskeleton in adults with chronic spinal cord injury. J Rehabil Med. 2017; 49(1):84–87. PMID: 27973679.
Article35. Nikander R, Sievänen H, Heinonen A, Daly RM, Uusi-Rasi K, Kannus P. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010; 8(1):47. PMID: 20663158.
Article36. Shanb AA, Youssef EF. The impact of adding weight-bearing exercise versus nonweight bearing programs to the medical treatment of elderly patients with osteoporosis. J Family Community Med. 2014; 21(3):176–181. PMID: 25374469.
Article37. Krassioukov A, Eng JJ, Claxton G, Sakakibara BM, Shum S. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010; 48(10):718–733. PMID: 20212501.
Article38. Peters HP, De Vries WR, Vanberge-Henegouwen GP, Akkermans LM. Potential benefits and hazards of physical activity and exercise on the gastrointestinal tract. Gut. 2001; 48(3):435–439. PMID: 11171839.
Article39. Iovino P, Chiarioni G, Bilancio G, Cirillo M, Mekjavic IB, Pisot R, et al. New onset of constipation during long-term physical inactivity: a proof-of-concept study on the immobility-induced bowel changes. PLoS One. 2013; 8(8):e72608. PMID: 23977327.
Article40. Gao R, Tao Y, Zhou C, Li J, Wang X, Chen L, et al. Exercise therapy in patients with constipation: a systematic review and meta-analysis of randomized controlled trials. Scand J Gastroenterol. 2019; 54(2):169–177. PMID: 30843436.
Article41. Chun A, Asselin PK, Knezevic S, Kornfeld S, Bauman WA, Korsten MA, et al. Changes in bowel function following exoskeletal-assisted walking in persons with spinal cord injury: an observational pilot study. Spinal Cord. 2020; 58(4):459–466. PMID: 31822808.
Article42. Frost F, Roach MJ, Kushner I, Schreiber P. Inflammatory C-reactive protein and cytokine levels in asymptomatic people with chronic spinal cord injury. Arch Phys Med Rehabil. 2005; 86(2):312–317. PMID: 15706560.
Article43. Gibson AE, Buchholz AC, Martin Ginis KA. SHAPE-SCI Research Group. C-reactive protein in adults with chronic spinal cord injury: increased chronic inflammation in tetraplegia vs paraplegia. Spinal Cord. 2008; 46(9):616–621. PMID: 18414426.
Article44. Turiel M, Sitia S, Cicala S, Magagnin V, Bo I, Porta A, et al. Robotic treadmill training improves cardiovascular function in spinal cord injury patients. Int J Cardiol. 2011; 149(3):323–329. PMID: 20219258.
Article45. Park G, Cho B, Kwon IS, Park BJ, Kim T, Cho KY, et al. Reliability and validity of Korean version of Falls Efficacy Scale-International (KFES-I). J Korean Acad Rehabil Med. 2010; 34(5):554–559.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Hope, Self-esteem and Quality of Life in People with Spinal Cord Injury
- Quality of Life in Spinal Cord Injured Persons
- Effects of Physical and Mental Health on the Quality of Life of People with Spinal Cord Injury
- Effect of Robotic-Assisted Gait Training in Patients With Incomplete Spinal Cord Injury
- Efficacy of Electrical Stimulation-Augmented Virtual Reality Training in Improving Balance in Individuals with Incomplete Spinal Cord Injury: Study Protocol of a Randomized Controlled Trial