Vimentin Deficiency Prevents High-Fat Diet-Induced Obesity and Insulin Resistance in Mice
- Affiliations
-
- 1Department of Molecular Medicine, College of Medicine, Ewha Womans University, Seoul, Korea.
- 2Immune and Vascular Cell Network Research Center, National Creative Initiatives, Department of Life Sciences, Ewha Womans University, Seoul, Korea.
- KMID: 2514078
- DOI: http://doi.org/10.4093/dmj.2019.0198
Abstract
Background Obesity and type 2 diabetes mellitus are world-wide health problems, and lack of understanding of their linking mechanism is one reason for limited treatment options. We determined if genetic deletion of vimentin, a type 3 intermediate filament, affects obesity and type 2 diabetes mellitus.
Methods We fed vimentin-null (
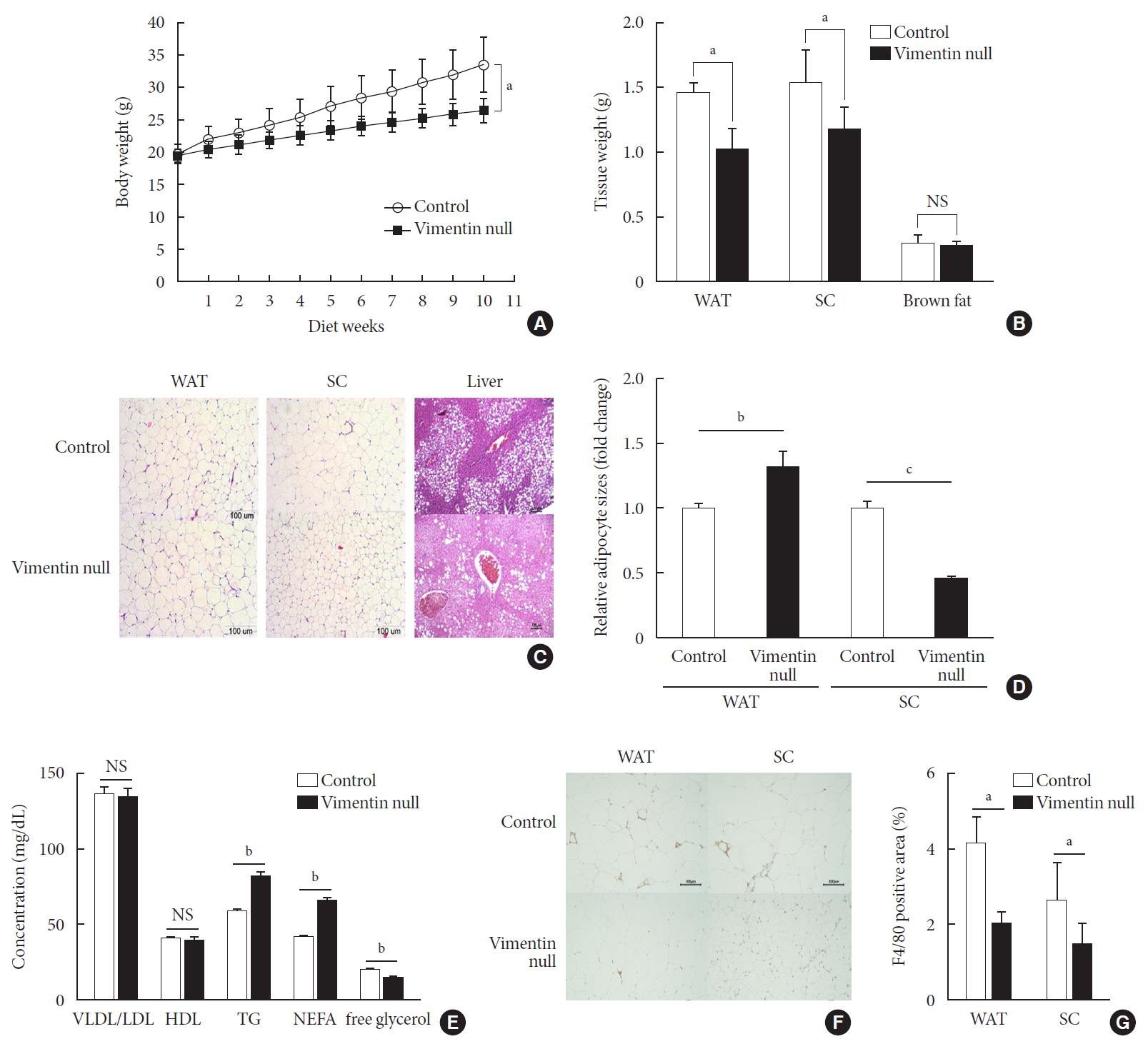
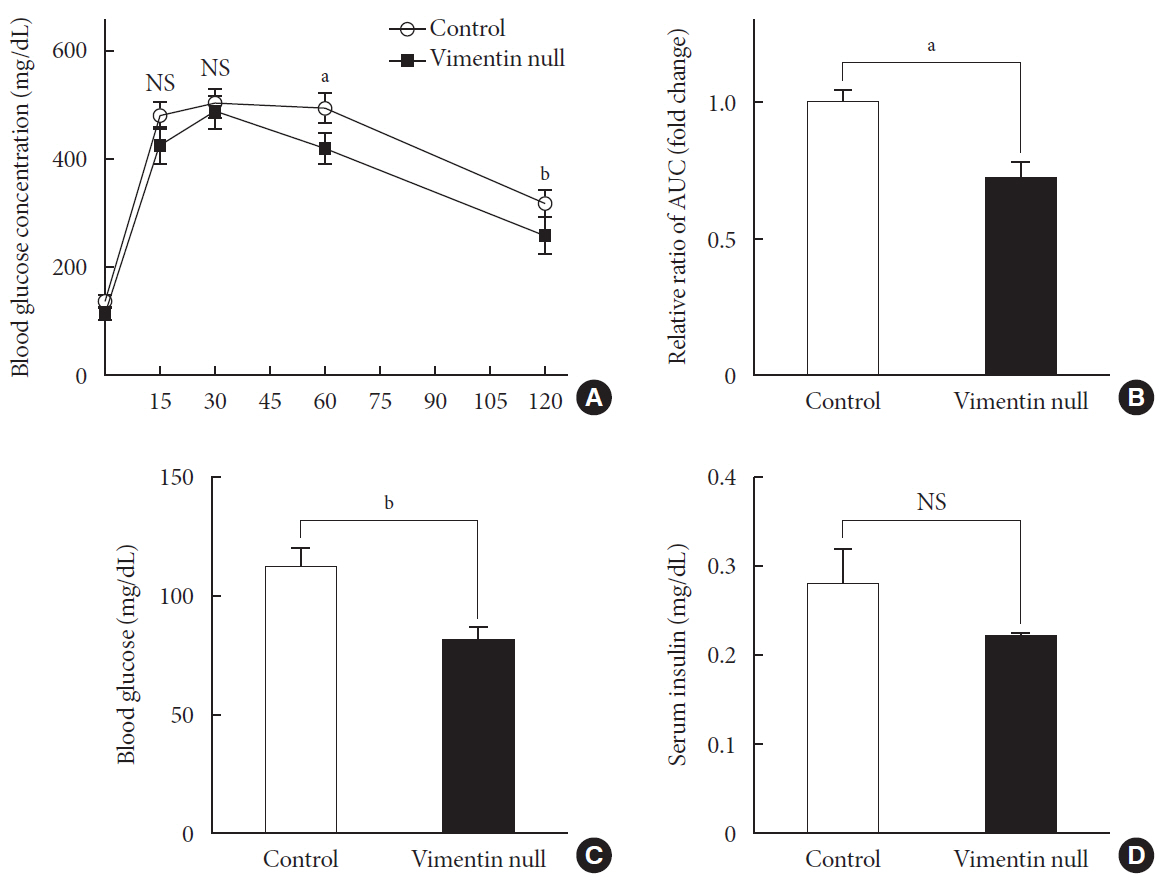
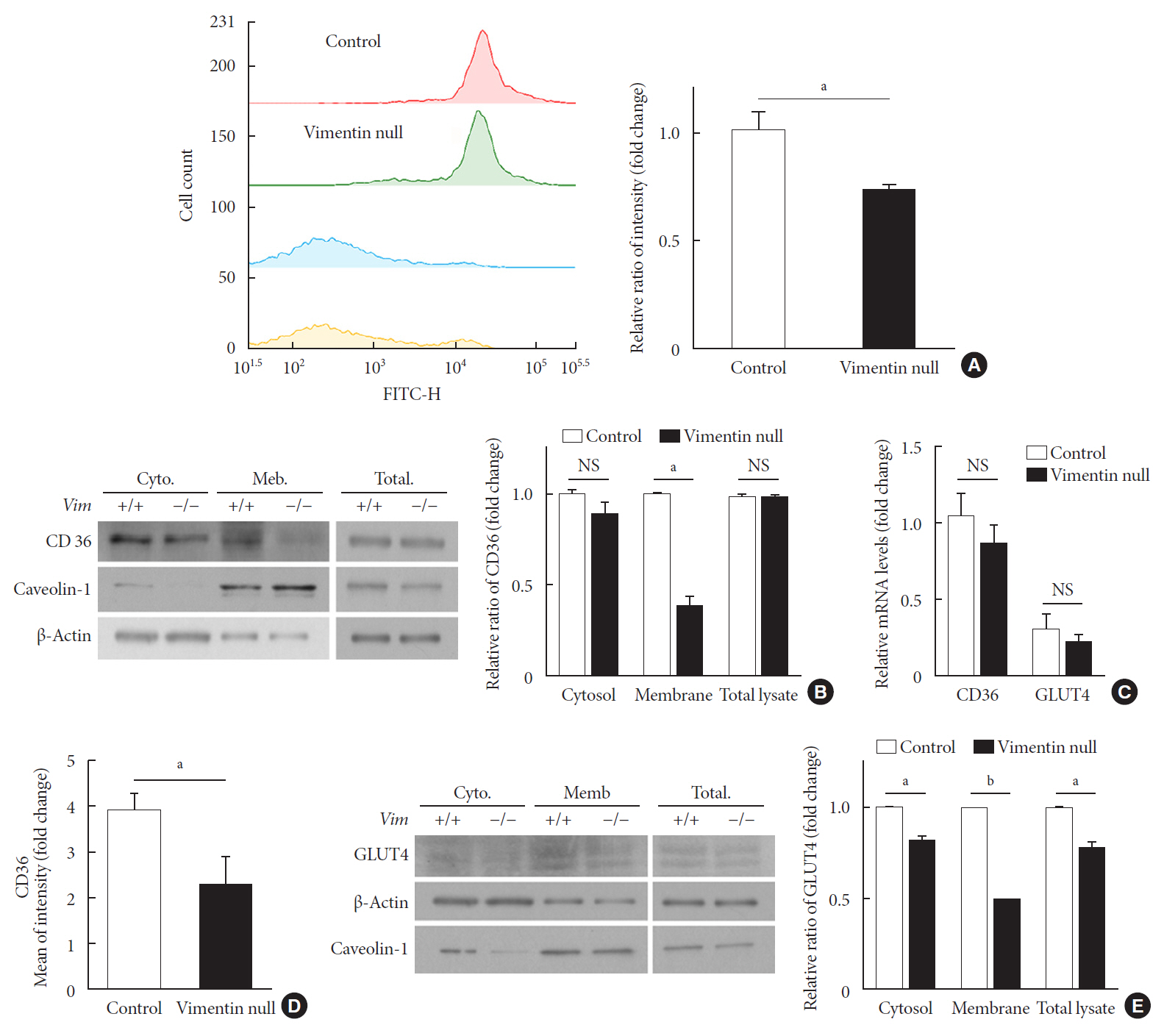
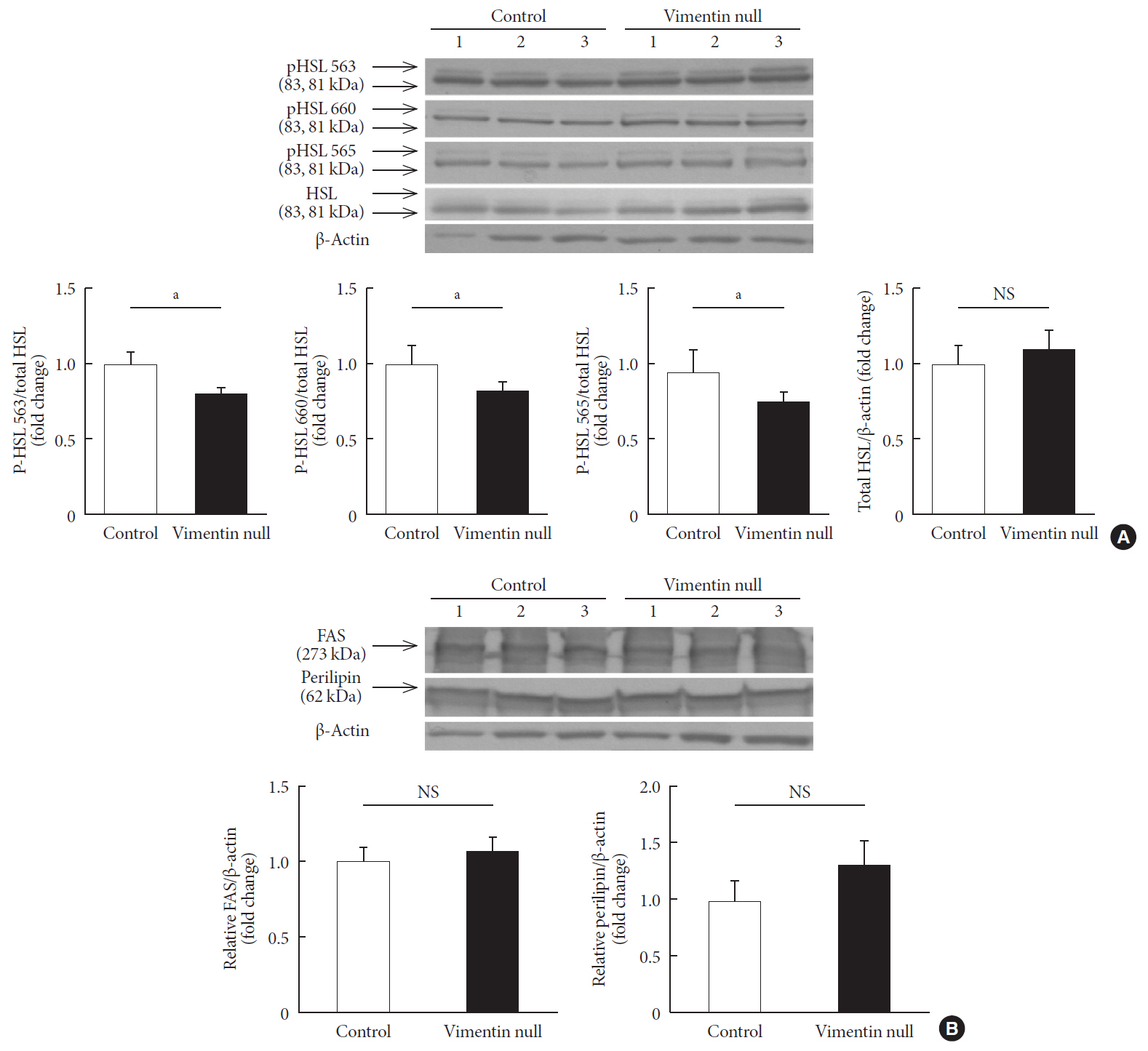
Vim −/−) mice and wild-type mice a high-fat diet (HFD) for 10 weeks and measured weight change, adiposity, blood lipids, and glucose. We performed intraperitoneal glucose tolerance tests and measured CD36, a major fatty acid translocase, and glucose transporter type 4 (GLUT4) in adipocytes from both groups of mice.Results Vim −/− mice fed an HFD showed less weight gain, less adiposity, improved glucose tolerance, and lower serum level of fasting glucose. However, serum triglyceride and non-esterified fatty acid levels were higher inVim −/− mice than in wild-type mice. Vimentin-null adipocytes showed 41.1% less CD36 on plasma membranes, 27% less uptake of fatty acids, and 50.3% less GLUT4, suggesting defects in intracellular trafficking of these molecules.Conclusion We concluded that vimentin deficiency prevents obesity and insulin resistance in mice fed an HFD and suggest vimentin as a central mediator linking obesity and type 2 diabetes mellitus.
Figure
Cited by 2 articles
-
The Role of Adipose Tissue Lipolysis in Diet-Induced Obesity: Focus on Vimentin
Eun Roh, Hye Jin Yoo
Diabetes Metab J. 2021;45(1):43-45. doi: 10.4093/dmj.2020.0293.Extracellular Vimentin Alters Energy Metabolism And Induces Adipocyte Hypertrophy
Ji-Hae Park, Soyeon Kwon, Young Mi Park
Diabetes Metab J. 2024;48(2):215-230. doi: 10.4093/dmj.2022.0332.
Reference
-
1. GBD 2015 Obesity Collaborators. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. 2017; 377:13–27.
Article2. Ye J. Mechanisms of insulin resistance in obesity. Front Med. 2013; 7:14–24.
Article3. Guo M, Ehrlicher AJ, Mahammad S, Fabich H, Jensen MH, Moore JR, et al. The role of vimentin intermediate filaments in cortical and cytoplasmic mechanics. Biophys J. 2013; 105:1562–1568.
Article4. Biskou O, Casanova V, Hooper KM, Kemp S, Wright GP, Satsangi J, et al. The type III intermediate filament vimentin regulates organelle distribution and modulates autophagy. PLoS One. 2019; 14:e0209665.
Article5. Liu CY, Lin HH, Tang MJ, Wang YK. Vimentin contributes to epithelial-mesenchymal transition cancer cell mechanics by mediating cytoskeletal organization and focal adhesion maturation. Oncotarget. 2015; 6:15966–15983.
Article6. Heid H, Rickelt S, Zimbelmann R, Winter S, Schumacher H, Dorflinger Y, et al. On the formation of lipid droplets in human adipocytes: the organization of the perilipin-vimentin cortex. PLoS One. 2014; 9:e90386.
Article7. Shen WJ, Patel S, Eriksson JE, Kraemer FB. Vimentin is a functional partner of hormone sensitive lipase and facilitates lipolysis. J Proteome Res. 2010; 9:1786–1794.
Article8. Hirata Y, Hosaka T, Iwata T, Le CT, Jambaldorj B, Teshigawara K, et al. Vimentin binds IRAP and is involved in GLUT4 vesicle trafficking. Biochem Biophys Res Commun. 2011; 405:96–101.
Article9. Wilhelmsson U, Stillemark-Billton P, Boren J, Pekny M. Vimentin is required for normal accumulation of body fat. Biol Chem. 2019; 400:1157–1162.
Article10. Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004; 53 Suppl 3:S215–S219.
Article11. Longo M, Zatterale F, Naderi J, Parrillo L, Formisano P, Raciti GA, et al. Adipose tissue dysfunction as determinant of obesity-associated metabolic complications. Int J Mol Sci. 2019; 20:E2358.
Article12. Park YM. CD36, a scavenger receptor implicated in atherosclerosis. Exp Mol Med. 2014; 46:e99.
Article13. Govers R. Molecular mechanisms of GLUT4 regulation in adipocytes. Diabetes Metab. 2014; 40:400–410.
Article14. Krintel C, Morgelin M, Logan DT, Holm C. Phosphorylation of hormone-sensitive lipase by protein kinase A in vitro promotes an increase in its hydrophobic surface area. FEBS J. 2009; 276:4752–4762.15. Colucci-Guyon E, Portier MM, Dunia I, Paulin D, Pournin S, Babinet C. Mice lacking vimentin develop and reproduce without an obvious phenotype. Cell. 1994; 79:679–694.
Article16. Shen WJ, Zaidi SK, Patel S, Cortez Y, Ueno M, Azhar R, et al. Ablation of vimentin results in defective steroidogenesis. Endocrinology. 2012; 153:3249–3257.
Article17. Bjorndal B, Burri L, Staalesen V, Skorve J, Berge RK. Different adipose depots: their role in the development of metabolic syndrome and mitochondrial response to hypolipidemic agents. J Obes. 2011; 2011:490650.18. Pepino MY, Love-Gregory L, Klein S, Abumrad NA. The fatty acid translocase gene CD36 and lingual lipase influence oral sensitivity to fat in obese subjects. J Lipid Res. 2012; 53:561–566.
Article19. Febbraio M, Silverstein RL. CD36: implications in cardiovascular disease. Int J Biochem Cell Biol. 2007; 39:2012–2030.
Article20. Oquendo P, Hundt E, Lawler J, Seed B. CD36 directly mediates cytoadherence of plasmodium falciparum parasitized erythrocytes. Cell. 1989; 58:95–101.
Article21. Griffin E, Re A, Hamel N, Fu C, Bush H, McCaffrey T, et al. A link between diabetes and atherosclerosis: glucose regulates expression of CD36 at the level of translation. Nat Med. 2001; 7:840–846.
Article22. Rac ME, Safranow K, Poncyljusz W. Molecular basis of human CD36 gene mutations. Mol Med. 2007; 13:288–296.
Article23. Nagy L, Tontonoz P, Alvarez JG, Chen H, Evans RM. Oxidized LDL regulates macrophage gene expression through ligand activation of PPARgamma. Cell. 1998; 93:229–240.24. Febbraio M, Abumrad NA, Hajjar DP, Sharma K, Cheng W, Pearce SF, et al. A null mutation in murine CD36 reveals an important role in fatty acid and lipoprotein metabolism. J Biol Chem. 1999; 274:19055–19062.
Article25. Abdoul-Azize S, Atek-Mebarki F, Bitam A, Sadou H, Koceir EA, Khan NA. Oro-gustatory perception of dietary lipids and calcium signaling in taste bud cells are altered in nutritionally obesity-prone Psammomys obesus. PLoS One. 2013; 8:e68532.
Article26. Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CD36-null mice. J Clin Invest. 2002; 109:1381–1389.
Article27. Furuhashi M, Ura N, Nakata T, Shimamoto K. Insulin sensitivity and lipid metabolism in human CD36 deficiency. Diabetes Care. 2003; 26:471–474.
Article28. Nath A, Li I, Roberts LR, Chan C. Elevated free fatty acid uptake via CD36 promotes epithelial-mesenchymal transition in hepatocellular carcinoma. Sci Rep. 2015; 5:14752.
Article29. Chang L, Chiang SH, Saltiel AR. Insulin signaling and the regulation of glucose transport. Mol Med. 2004; 10:65–71.
Article30. Asano T, Fujishiro M, Kushiyama A, Nakatsu Y, Yoneda M, Kamata H, et al. Role of phosphatidylinositol 3-kinase activation on insulin action and its alteration in diabetic conditions. Biol Pharm Bull. 2007; 30:1610–1616.
Article31. Huang S, Czech MP. The GLUT4 glucose transporter. Cell Metab. 2007; 5:237–252.
Article32. Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific overexpression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005; 289:E551–E561.
Article33. Atkinson BJ, Griesel BA, King CD, Josey MA, Olson AL. Moderate GLUT4 overexpression improves insulin sensitivity and fasting triglyceridemia in high-fat diet-fed transgenic mice. Diabetes. 2013; 62:2249–2258.
Article34. Roefs MM, Carlotti F, Jones K, Wills H, Hamilton A, Verschoor M, et al. Increased vimentin in human α- and β-cells in type 2 diabetes. J Endocrinol. 2017; 233:217–227.
Article35. Kumar N, Robidoux J, Daniel KW, Guzman G, Floering LM, Collins S. Requirement of vimentin filament assembly for beta3-adrenergic receptor activation of ERK MAP kinase and lipolysis. J Biol Chem. 2007; 282:9244–9250.36. Asensio C, Jimenez M, Kuhne F, Rohner-Jeanrenaud F, Muzzin P. The lack of beta-adrenoceptors results in enhanced insulin sensitivity in mice exhibiting increased adiposity and glucose intolerance. Diabetes. 2005; 54:3490–3495.37. Potokar M, Kreft M, Li L, Daniel Andersson J, Pangrsic T, Chowdhury HH, et al. Cytoskeleton and vesicle mobility in astrocytes. Traffic. 2007; 8:12–20.
Article38. Samovski D, Sun J, Pietka T, Gross RW, Eckel RH, Su X, et al. Regulation of AMPK activation by CD36 links fatty acid uptake to β-oxidation. Diabetes. 2015; 64:353–359.
Article39. Zhou D, Samovski D, Okunade AL, Stahl PD, Abumrad NA, Su X. CD36 level and trafficking are determinants of lipolysis in adipocytes. FASEB J. 2012; 26:4733–4742.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Blood Sugar Control and Low-Carbohydrate High-Fat Diet
- Anti-obesity and LDL-cholesterol lowering effects of silkworm hemolymph in C57BL/6N mice fed high fat diet
- Ameliorative effect of myricetin on insulin resistance in mice fed a high-fat, high-sucrose diet
- Sasa borealis leaves extract improves insulin resistance by modulating inflammatory cytokine secretion in high fat diet-induced obese C57/BL6J mice
- Effect of Exercise Training on Insulin Sensitivity and Intracellular Glucose Metabolism in Skeletal Muscle of High Fat-fed Rats