Notch1 Has an Important Role in β-Cell Mass Determination and Development of Diabetes
- Affiliations
-
- 1Department of Internal Medicine, Gachon University Gil Medical Center, Gachon University College of Medicine, Incheon, Korea.
- 2Department of Internal Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- 3Institute of Clinical Medicine, Gachon University Gil Medical Center, Incheon, Korea.
- KMID: 2514077
- DOI: http://doi.org/10.4093/dmj.2019.0160
Abstract
Background Notch signaling pathway plays an important role in regulating pancreatic endocrine and exocrine cell fate during pancreas development. Notch signaling is also expressed in adult pancreas. There are few studies on the effect of Notch on adult pancreas. Here, we investigated the role of Notch in islet mass and glucose homeostasis in adult pancreas using Notch1 antisense transgenic (NAS).
Methods Western blot analysis was performed for the liver of 8-week-old male NAS mice. We also conducted an intraperitoneal glucose tolerance test (IPGTT) and intraperitoneal insulin tolerance test in 8-week-old male NAS mice and male C57BL/6 mice (control). Morphologic observation of pancreatic islet and β-cell was conducted in two groups. Insulin secretion capacity in islets was measured by glucose-stimulated insulin secretion (GSIS) and perifusion.
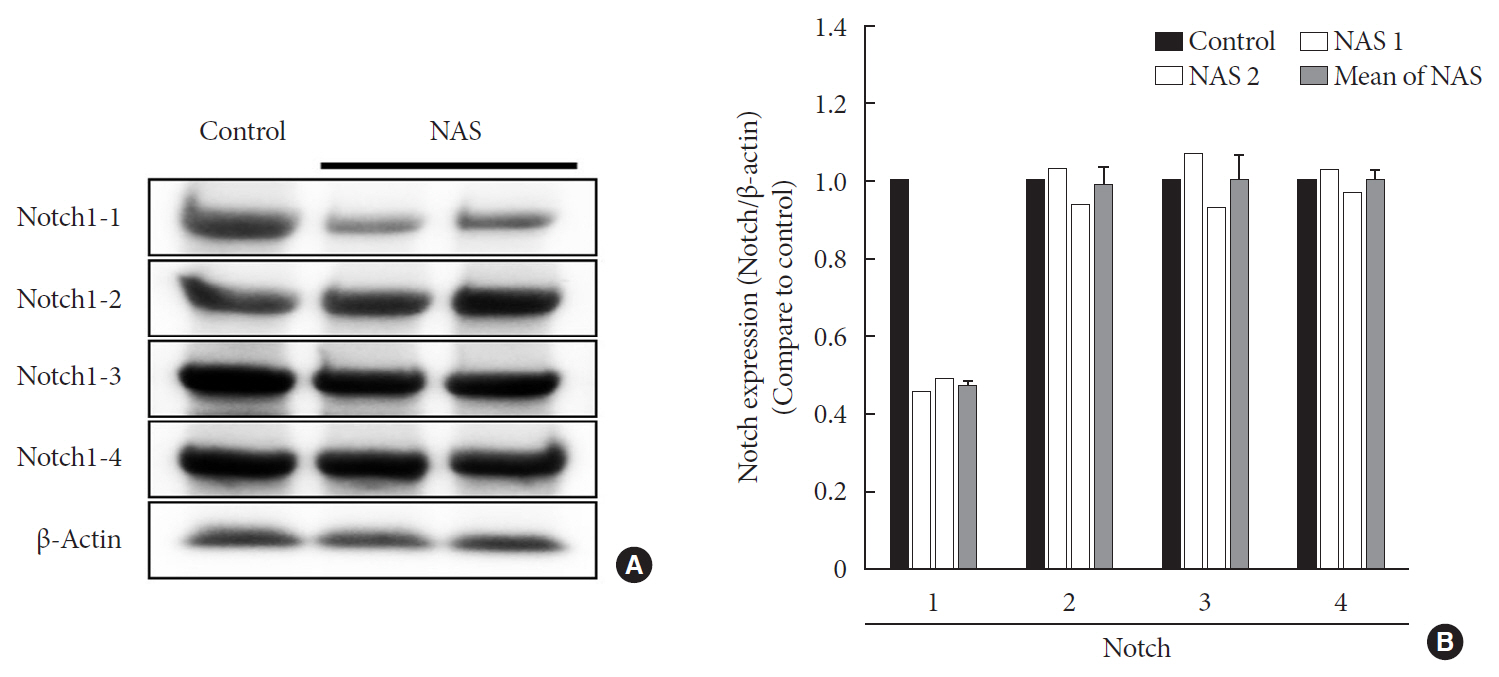
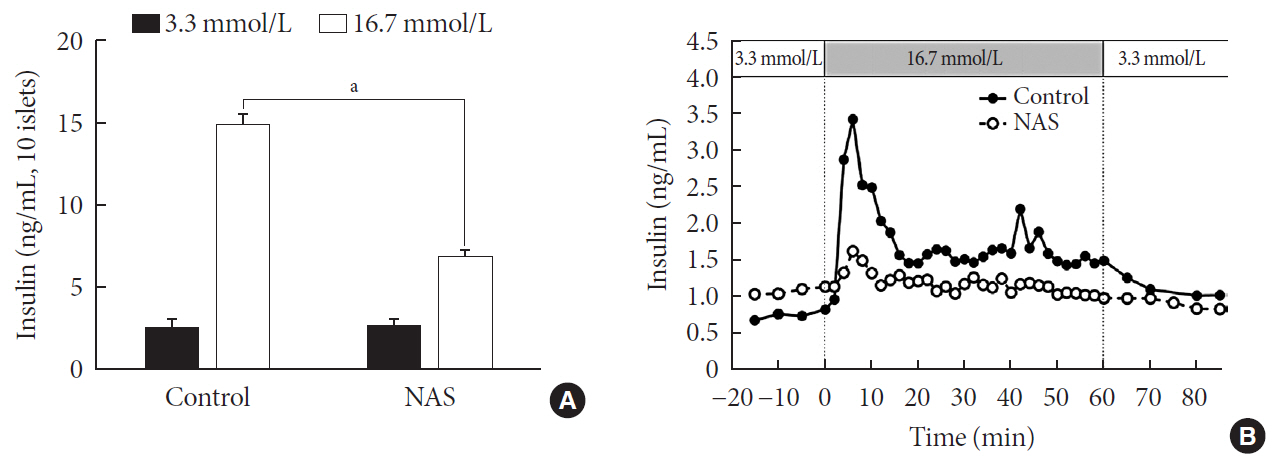
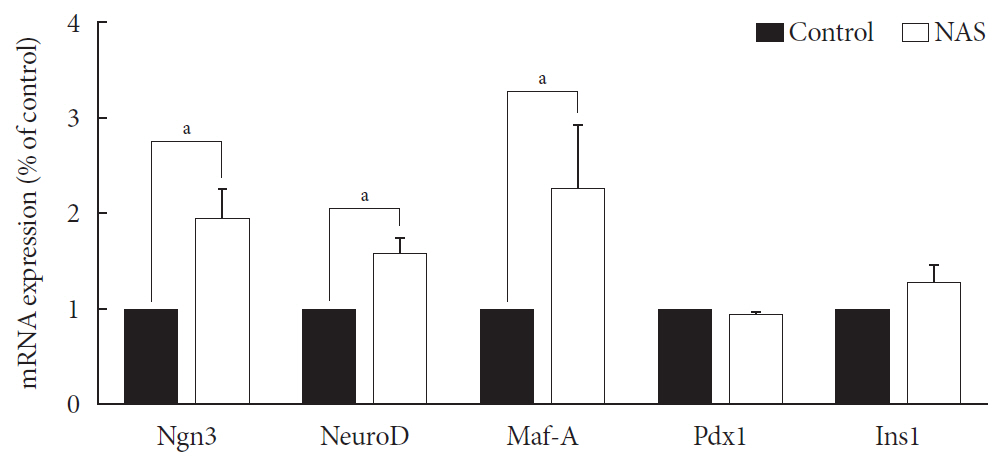
Results NAS mice showed higher glucose levels and lower insulin secretion in IPGTT than the control mice. There was no significant difference in insulin resistance. Total islet and β-cell masses were decreased in NAS mice. The number of large islets (≥250 µm) decreased while that of small islets (<250 µm) increased. Reduced insulin secretion was observed in GSIS and perifusion. Neurogenin3, neurogenic differentiation, and MAF bZIP transcription factor A levels increased in NAS mice.
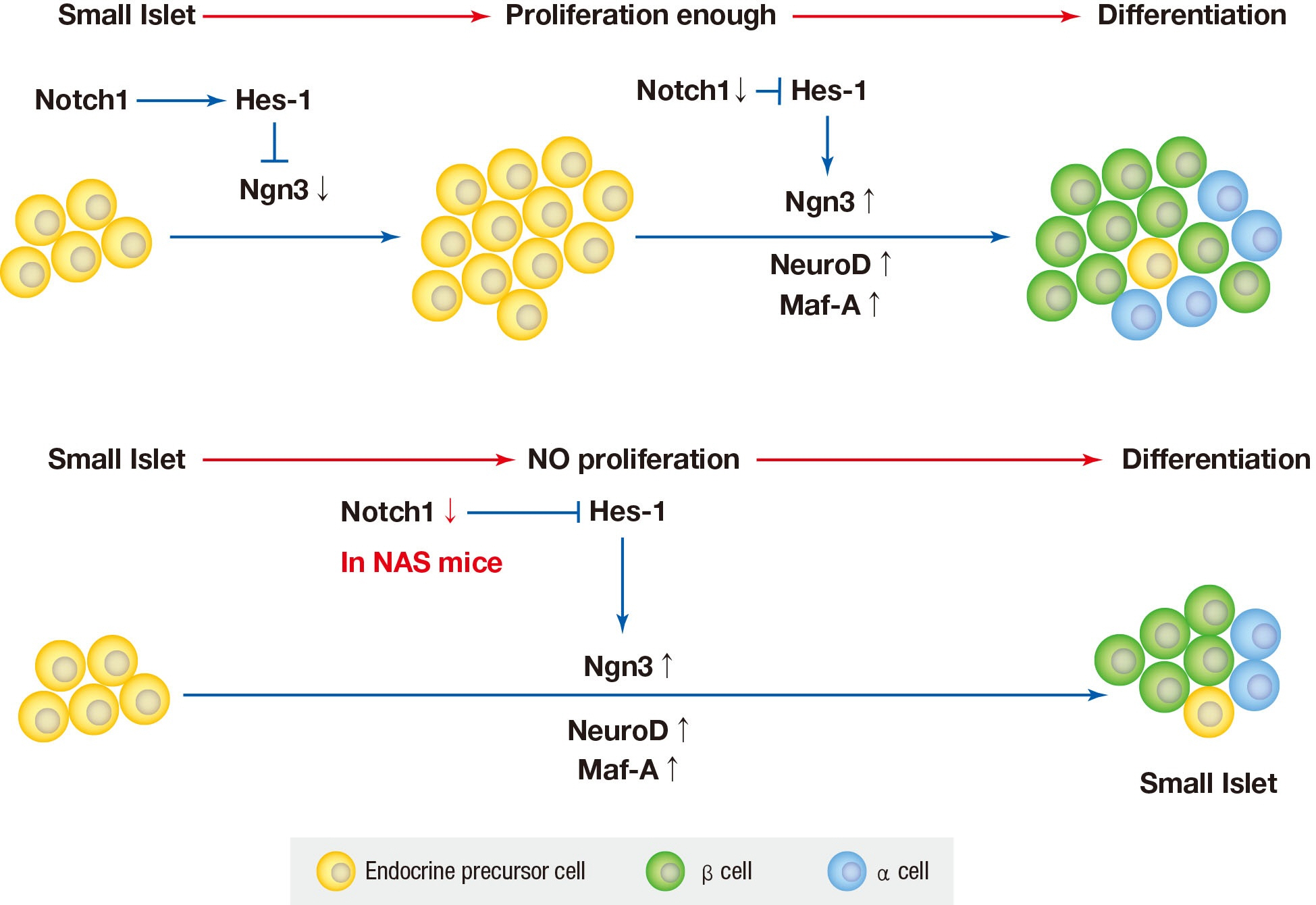
Conclusion Our study provides that Notch1 inhibition decreased insulin secretion and decreased islet and β-cell masses. It is thought that Notch1 inhibition suppresses islet proliferation and induces differentiation of small islets. In conclusion, Notch signaling pathway may play an important role in β-cell mass determination and diabetes.
Figure
Reference
-
1. American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes-2018. Diabetes Care. 2018; 41:S13–S27.2. Cernea S, Dobreanu M. Diabetes and beta cell function: from mechanisms to evaluation and clinical implications. Biochem Med (Zagreb). 2013; 23:266–280.
Article3. Chen C, Cohrs CM, Stertmann J, Bozsak R, Speier S. Human beta cell mass and function in diabetes: recent advances in knowledge and technologies to understand disease pathogenesis. Mol Metab. 2017; 6:943–957.
Article4. Karadimos MJ, Kapoor A, El Khattabi I, Sharma A. β-Cell preservation and regeneration for diabetes treatment: where are we now? Diabetes Manag (Lond). 2012; 2:213–222.
Article5. Naujok O, Burns C, Jones PM, Lenzen S. Insulin-producing surrogate β-cells from embryonic stem cells: are we there yet? Mol Ther. 2011; 19:1759–1768.
Article6. Apelqvist A, Li H, Sommer L, Beatus P, Anderson DJ, Honjo T, et al. Notch signalling controls pancreatic cell differentiation. Nature. 1999; 400:877–881.
Article7. Murtaugh LC, Stanger BZ, Kwan KM, Melton DA. Notch signaling controls multiple steps of pancreatic differentiation. Proc Natl Acad Sci U S A. 2003; 100:14920–14925.
Article8. Kim W, Shin YK, Kim BJ, Egan JM. Notch signaling in pancreatic endocrine cell and diabetes. Biochem Biophys Res Commun. 2010; 392:247–251.
Article9. Gridley T. Notch signaling and inherited disease syndromes. Hum Mol Genet. 2003; 12 Spec No 1:R9–R13.
Article10. Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, et al. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000; 14:1343–1352.11. Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, et al. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999; 8:723–730.
Article12. Cheng P, Zlobin A, Volgina V, Gottipati S, Osborne B, Simel EJ, et al. Notch-1 regulates NF-kappaB activity in hemopoietic progenitor cells. J Immunol. 2001; 167:4458–4467.13. Shelly LL, Fuchs C, Miele L. Notch-1 inhibits apoptosis in murine erythroleukemia cells and is necessary for differentiation induced by hybrid polar compounds. J Cell Biochem. 1999; 73:164–175.
Article14. Kopinke D, Brailsford M, Pan FC, Magnuson MA, Wright CV, Murtaugh LC. Ongoing Notch signaling maintains phenotypic fidelity in the adult exocrine pancreas. Dev Biol. 2012; 362:57–64.
Article15. Bartolome A, Zhu C, Sussel L, Pajvani UB. Notch signaling dynamically regulates adult β cell proliferation and maturity. J Clin Invest. 2019; 129:268–280.
Article16. Suzuki K, Bonner-Weir S, Hollister J, Weir GC. A method for estimating number and mass of islets transplanted within a membrane device. Cell Transplant. 1996; 5:613–625.
Article17. Wang RN, Kloppel G, Bouwens L. Duct- to islet-cell differentiation and islet growth in the pancreas of duct-ligated adult rats. Diabetologia. 1995; 38:1405–1411.
Article18. Rosenberg L, Brown RA, Duguid WP. A new approach to the induction of duct epithelial hyperplasia and nesidioblastosis by cellophane wrapping of the hamster pancreas. J Surg Res. 1983; 35:63–72.
Article19. Rosenberg L, Duguid WP, Healy M, Clas D, Vinik AI. Reversal of diabetes by the induction of islet cell neogenesis. Transplant Proc. 1992; 24:1027–1028.20. Finegood DT, Scaglia L, Bonner-Weir S. Dynamics of beta-cell mass in the growing rat pancreas. Estimation with a simple mathematical model. Diabetes. 1995; 44:249–256.
Article21. Fujimoto WY, Metz SA. Phasic glucose-stimulated insulin secretion by neonatal rat pancreatic islet cells. Enhancement by sodium salicylate. Diabetes. 1984; 33:872–878.
Article22. Bergstrom RW, Fujimoto WY, Teller DC, de Haen C. Oscillatory insulin secretion in perifused isolated rat islets. Am J Physiol. 1989; 257:E479–E485.
Article23. Aguayo-Mazzucato C, Bonner-Weir S. Stem cell therapy for type 1 diabetes mellitus. Nat Rev Endocrinol. 2010; 6:139–148.
Article24. Juhl K, Bonner-Weir S, Sharma A. Regenerating pancreatic beta-cells: plasticity of adult pancreatic cells and the feasibility of in-vivo neogenesis. Curr Opin Organ Transplant. 2010; 15:79–85.25. Hanley NA, Hanley KP, Miettinen PJ, Otonkoski T. Weighing up beta-cell mass in mice and humans: self-renewal, progenitors or stem cells? Mol Cell Endocrinol. 2008; 288:79–85.26. Talchai C, Xuan S, Lin HV, Sussel L, Accili D. Pancreatic β cell dedifferentiation as a mechanism of diabetic β cell failure. Cell. 2012; 150:1223–1234.
Article27. Accili D, Talchai SC, Kim-Muller JY, Cinti F, Ishida E, Ordelheide AM, et al. When β-cells fail: lessons from dedifferentiation. Diabetes Obes Metab. 2016; 18 Suppl 1:117–122.
Article28. Pajvani UB, Shawber CJ, Samuel VT, Birkenfeld AL, Shulman GI, Kitajewski J, et al. Inhibition of Notch signaling ameliorates insulin resistance in a FoxO1-dependent manner. Nat Med. 2011; 17:961–967.
Article29. Edlund H. Pancreatic organogenesis: developmental mechanisms and implications for therapy. Nat Rev Genet. 2002; 3:524–532.30. Wang H, Brun T, Kataoka K, Sharma AJ, Wollheim CB. MAFA controls genes implicated in insulin biosynthesis and secretion. Diabetologia. 2007; 50:348–358.
Article31. Aramata S, Han SI, Kataoka K. Roles and regulation of transcription factor MafA in islet beta-cells. Endocr J. 2007; 54:659–666.32. Yang YP, Thorel F, Boyer DF, Herrera PL, Wright CV. Context-specific α- to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011; 25:1680–1685.33. Ahnfelt-Ronne J, Hald J, Bodker A, Yassin H, Serup P, Hecksher-Sorensen J. Preservation of proliferating pancreatic progenitor cells by delta-Notch signaling in the embryonic chicken pancreas. BMC Dev Biol. 2007; 7:63.
Article34. Hart A, Papadopoulou S, Edlund H. Fgf10 maintains notch activation, stimulates proliferation, and blocks differentiation of pancreatic epithelial cells. Dev Dyn. 2003; 228:185–193.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Significance of Notch Expression in Acute Myeloid Leukemia
- The Role of Notch1 Signaling in Anaplastic Thyroid Carcinoma
- High Notch1 Expression Correlates with Tumor Stage and Size in Clear Cell Renal Cell Carcinoma
- Alteration of NOTCH1 in T-cell Acute Lymphoblastic Leukemia and Development of Target Therapeutic Agent
- Notch1 in Tumor Microvascular Endothelial Cells and Tumoral miR-34a as Prognostic Markers in Locally Advanced Triple-Negative Breast Cancer