J Korean Med Sci.
2021 Mar;36(8):e58. 10.3346/jkms.2021.36.e58.
Comprehensive Analysis of the Effect of Probiotic Intake by the Mother on Human Breast Milk and Infant Fecal Microbiota
- Affiliations
-
- 1Department of Pediatrics, Chung-Ang University Hospital, Seoul, Korea
- 2InfoBoss Inc., Seoul, Korea
- 3Department of Pediatrics, College of Medicine, Chung-Ang University, Seoul, Korea
- KMID: 2513533
- DOI: http://doi.org/10.3346/jkms.2021.36.e58
Abstract
- Background
Human breast milk (HBM) contains optimal nutrients for infant growth. Probiotics are used to prevent disease and, when taken by the mother, they may affect infant microbiome as well as HBM. However, few studies have specifically investigated the effect of probiotic intake by the mother on HBM and infant microbiota at genus/species level. Therefore, we present a comprehensive analysis of paired HBM and infant feces (IF) microbiome samples before and after probiotic intake by HBM-producing mothers.
Methods
Lactating mothers were administered with Lactobacillus rhamnosus (n = 9) or Saccharomyces boulardii capsules (n = 9), for 2 months; or no probiotic (n = 7). Paired HBM and IF samples were collected before and after treatment and analyzed by next-generation sequencing.
Results
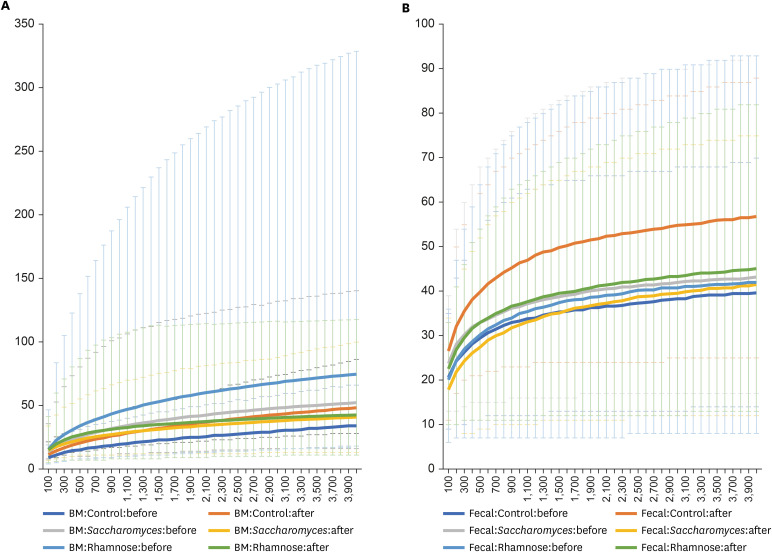
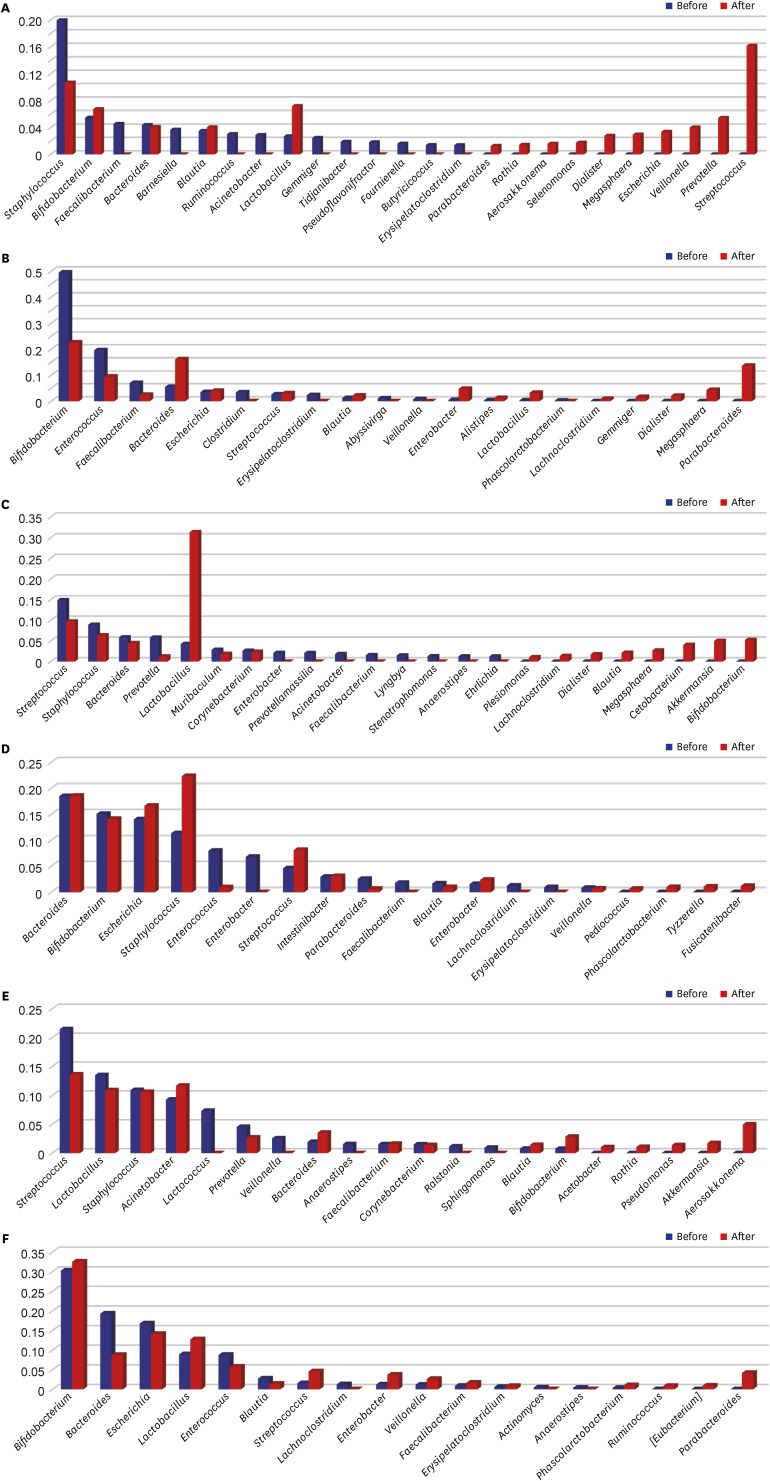
Forty-three HBM and 49 IF samples were collected and sequenced. Overall, in 43 HBM samples, 1,190 microbial species belonging to 684 genera, 245 families, 117 orders, and 56 classes were detected. In 49 IF samples, 372 microbial species belonging to 195 genera, 79 families, 42 orders, and 18 classes were identified. Eight of 20 most abundant genera in both HBM and IF samples overlapped: Streptococcus (14.42%), Lactobacillus, Staphylococcus, and Veillonella, which were highly abundant in the HBM samples; and Bifidobacterium (27.397%), Bacteroides, and Faecalibacterium, which were highly abundant in the IF samples. Several major bacterial genera and species were detected in the HBM and IF samples after probiotic treatment, illustrating complex changes in the microbiomes upon treatment.
Conclusion
This is the first Korean microbiome study in which the effect of different probiotic intake by the mother on the microbiota in HBM and IF samples was investigated. This study provides a cornerstone to further the understanding of the effect of probiotics on the mother and infant microbiomes.
Keyword
Figure
Reference
-
1. McDowell MM, Wang CY, Kennedy-Stephenson J. Breastfeeding in the United States: findings from the national health and nutrition examination surveys, 1999–2006. NCHS Data Brief. 2008; (5):1–8.
Article2. Centers for Disease Control and Prevention. Breastfeeding among US children. Updated 2020. Accessed May 28, 2020. https://www.cdc.gov/breastfeeding/about-breastfeeding/index.html.3. Centers for Disease Control and Prevention. CDC national survey of maternity practices in infant nutrition and care (mPINC). Updated 2020. Accessed July 2, 2020. https://www.cdc.gov/breastfeeding/data/mpinc.4. Arora T, Singh S, Sharma RK. Probiotics: interaction with gut microbiome and antiobesity potential. Nutrition. 2013; 29(4):591–596. PMID: 23287068.
Article5. Gao Z, Guo B, Gao R, Zhu Q, Wu W, Qin H. Probiotics modify human intestinal mucosa-associated microbiota in patients with colorectal cancer. Mol Med Rep. 2015; 12(4):6119–6127. PMID: 26238090.
Article6. Singh RK, Chang HW, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med. 2017; 15(1):73. PMID: 28388917.
Article7. Vitetta L, Coulson S, Linnane AW, Butt H. The gastrointestinal microbiome and musculoskeletal diseases: a beneficial role for probiotics and prebiotics. Pathogens. 2013; 2(4):606–626. PMID: 25437335.
Article8. Lee SY, Yu J, Ahn KM, Kim KW, Shin YH, Lee KS, et al. Additive effect between IL-13 polymorphism and cesarean section delivery/prenatal antibiotics use on atopic dermatitis: a birth cohort study (COCOA). PLoS One. 2014; 9(5):e96603. PMID: 24848505.
Article9. Lee JY, Seo JH, Kwon JW, Yu J, Kim BJ, Lee SY, et al. Exposure to gene-environment interactions before 1 year of age may favor the development of atopic dermatitis. Int Arch Allergy Immunol. 2012; 157(4):363–371. PMID: 22123373.
Article10. Fujimura KE, Sitarik AR, Havstad S, Lin DL, Levan S, Fadrosh D, et al. Neonatal gut microbiota associates with childhood multisensitized atopy and T cell differentiation. Nat Med. 2016; 22(10):1187–1191. PMID: 27618652.
Article11. Alam C, Bittoun E, Bhagwat D, Valkonen S, Saari A, Jaakkola U, et al. Effects of a germ-free environment on gut immune regulation and diabetes progression in non-obese diabetic (NOD) mice. Diabetologia. 2011; 54(6):1398–1406. PMID: 21380595.
Article12. Xu WT, Nie YZ, Yang Z, Lu NH. The crosstalk between gut microbiota and obesity and related metabolic disorders. Future Microbiol. 2016; 11(6):825–836. PMID: 27192213.
Article13. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009; 457(7228):480–484. PMID: 19043404.
Article14. Arrieta MC, Stiemsma LT, Dimitriu PA, Thorson L, Russell S, Yurist-Doutsch S, et al. Early infancy microbial and metabolic alterations affect risk of childhood asthma. Sci Transl Med. 2015; 7(307):307ra152.
Article15. Xuan C, Shamonki JM, Chung A, Dinome ML, Chung M, Sieling PA, et al. Microbial dysbiosis is associated with human breast cancer. PLoS One. 2014; 9(1):e83744. PMID: 24421902.
Article16. Cremon C, Barbaro MR, Ventura M, Barbara G. Pre- and probiotic overview. Curr Opin Pharmacol. 2018; 43:87–92. PMID: 30219638.
Article17. Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2007; 85(3):816–823. PMID: 17344505.
Article18. Sanaie S, Ebrahimi-Mameghani M, Mahmoodpoor A, Shadvar K, Golzari SE. Effect of a probiotic preparation (VSL#3) on cardiovascularrisk parameters in critically-ill patients. J Cardiovasc Thorac Res. 2013; 5(2):67–70. PMID: 24251014.19. Gupta N, Kumar A, Sharma P, Garg V, Sharma BC, Sarin SK. Effects of the adjunctive probiotic VSL#3 on portal haemodynamics in patients with cirrhosis and large varices: a randomized trial. Liver Int. 2013; 33(8):1148–1157. PMID: 23601333.
Article20. Perez PF, Doré J, Leclerc M, Levenez F, Benyacoub J, Serrant P, et al. Bacterial imprinting of the neonatal immune system: lessons from maternal cells? Pediatrics. 2007; 119(3):e724–32. PMID: 17332189.
Article21. Jiménez E, Fernández L, Maldonado A, Martín R, Olivares M, Xaus J, et al. Oral administration of Lactobacillus strains isolated from breast milk as an alternative for the treatment of infectious mastitis during lactation. Appl Environ Microbiol. 2008; 74(15):4650–4655. PMID: 18539795.22. Arroyo R, Martín V, Maldonado A, Jiménez E, Fernández L, Rodríguez JM. Treatment of infectious mastitis during lactation: antibiotics versus oral administration of Lactobacilli isolated from breast milk. Clin Infect Dis. 2010; 50(12):1551–1558. PMID: 20455694.
Article23. Jost T, Lacroix C, Braegger CP, Rochat F, Chassard C. Vertical mother-neonate transfer of maternal gut bacteria via breastfeeding. Environ Microbiol. 2014; 16(9):2891–2904. PMID: 24033881.
Article24. Abrahamsson TR, Sinkiewicz G, Jakobsson T, Fredrikson M, Björkstén B. Probiotic lactobacilli in breast milk and infant stool in relation to oral intake during the first year of life. J Pediatr Gastroenterol Nutr. 2009; 49(3):349–354. PMID: 19525871.
Article25. Fernández L, Langa S, Martín V, Maldonado A, Jiménez E, Martín R, et al. The human milk microbiota: origin and potential roles in health and disease. Pharmacol Res. 2013; 69(1):1–10. PMID: 22974824.
Article26. La Rosa PS, Warner BB, Zhou Y, Weinstock GM, Sodergren E, Hall-Moore CM, et al. Patterned progression of bacterial populations in the premature infant gut. Proc Natl Acad Sci U S A. 2014; 111(34):12522–12527. PMID: 25114261.
Article27. Azad MB, Konya T, Maughan H, Guttman DS, Field CJ, Chari RS, et al. Gut microbiota of healthy Canadian infants: profiles by mode of delivery and infant diet at 4 months. CMAJ. 2013; 185(5):385–394. PMID: 23401405.
Article28. Kim G, Bae J, Kim MJ, Kwon H, Park G, Kim SJ, et al. Delayed establishment of gut microbiota in infants delivered by cesarean section. Front Microbiol. 2020; 11:2099. PMID: 33013766.
Article29. Madan JC, Hoen AG, Lundgren SN, Farzan SF, Cottingham KL, Morrison HG, et al. Association of cesarean delivery and formula supplementation with the intestinal microbiome of 6-week-old infants. JAMA Pediatr. 2016; 170(3):212–219. PMID: 26752321.
Article30. Lax S, Smith DP, Hampton-Marcell J, Owens SM, Handley KM, Scott NM, et al. Longitudinal analysis of microbial interaction between humans and the indoor environment. Science. 2014; 345(6200):1048–1052. PMID: 25170151.
Article31. Gomez-Llorente C, Plaza-Diaz J, Aguilera M, Muñoz-Quezada S, Bermudez-Brito M, Peso-Echarri P, et al. Three main factors define changes in fecal microbiota associated with feeding modality in infants. J Pediatr Gastroenterol Nutr. 2013; 57(4):461–466. PMID: 23752082.
Article32. Magoč T, Salzberg SL. FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics. 2011; 27(21):2957–2963. PMID: 21903629.
Article33. Li W, Godzik A. Cd-hit: a fast program for clustering and comparing large sets of protein or nucleotide sequences. Bioinformatics. 2006; 22(13):1658–1659. PMID: 16731699.
Article34. Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990; 215(3):403–410. PMID: 2231712.
Article35. Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7(5):335–336. PMID: 20383131.
Article36. Cabrera-Rubio R, Collado MC, Laitinen K, Salminen S, Isolauri E, Mira A. The human milk microbiome changes over lactation and is shaped by maternal weight and mode of delivery. Am J Clin Nutr. 2012; 96(3):544–551. PMID: 22836031.
Article37. Murphy K, Curley D, O'Callaghan TF, O'Shea CA, Dempsey EM, O’Toole PW, et al. The composition of human milk and infant faecal microbiota over the first three months of life: a pilot study. Sci Rep. 2017; 7(1):40597. PMID: 28094284.
Article38. Heikkilä MP, Saris PE. Inhibition of Staphylococcus aureus by the commensal bacteria of human milk. J Appl Microbiol. 2003; 95(3):471–478. PMID: 12911694.39. Jost T, Lacroix C, Braegger C, Chassard C. Assessment of bacterial diversity in breast milk using culture-dependent and culture-independent approaches. Br J Nutr. 2013; 110(7):1253–1262. PMID: 23507238.
Article40. Martín R, Heilig HG, Zoetendal EG, Jiménez E, Fernández L, Smidt H, et al. Cultivation-independent assessment of the bacterial diversity of breast milk among healthy women. Res Microbiol. 2007; 158(1):31–37. PMID: 17224259.
Article41. Turroni F, Foroni E, Pizzetti P, Giubellini V, Ribbera A, Merusi P, et al. Exploring the diversity of the bifidobacterial population in the human intestinal tract. Appl Environ Microbiol. 2009; 75(6):1534–1545. PMID: 19168652.
Article42. Fanaro S, Vigi V, Chierici R, Boehm G. Fecal flora measurements of breastfed infants using an integrated transport and culturing system. Acta Paediatr. 2003; 92(5):634–635. PMID: 12839299.
Article43. Favier CF, Vaughan EE, De Vos WM, Akkermans AD. Molecular monitoring of succession of bacterial communities in human neonates. Appl Environ Microbiol. 2002; 68(1):219–226. PMID: 11772630.
Article44. Martín V, Maldonado-Barragán A, Moles L, Rodriguez-Baños M, Campo RD, Fernández L, et al. Sharing of bacterial strains between breast milk and infant feces. J Hum Lact. 2012; 28(1):36–44. PMID: 22267318.
Article45. Hunt KM, Foster JA, Forney LJ, Schütte UM, Beck DL, Abdo Z, et al. Characterization of the diversity and temporal stability of bacterial communities in human milk. PLoS One. 2011; 6(6):e21313. PMID: 21695057.
Article46. Ward TL, Hosid S, Ioshikhes I, Altosaar I. Human milk metagenome: a functional capacity analysis. BMC Microbiol. 2013; 13(1):116. PMID: 23705844.
Article47. Martín R, Langa S, Reviriego C, Jimínez E, Marín ML, Xaus J, et al. Human milk is a source of lactic acid bacteria for the infant gut. J Pediatr. 2003; 143(6):754–758. PMID: 14657823.
Article48. Song YL, Liu CX, McTeague M, Finegold SM. “Bacteroides nordii” sp. nov. and “Bacteroides salyersae” sp. nov. isolated from clinical specimens of human intestinal origin. J Clin Microbiol. 2004; 42(12):5565–5570. PMID: 15583282.49. Lagkouvardos I, Pukall R, Abt B, Foesel BU, Meier-Kolthoff JP, Kumar N, et al. The mouse intestinal bacterial collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol. 2016; 1(10):16131. PMID: 27670113.
Article50. Coppa GV, Bruni S, Morelli L, Soldi S, Gabrielli O. The first prebiotics in humans: human milk oligosaccharides. J Clin Gastroenterol. 2004; 38(6):Suppl. S80–3. PMID: 15220665.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concept Analysis of Effective Breastfeeding
- Immediate systemic allergic reaction in an infant to fish allergen ingested through breast milk
- Components of human breast milk: from macronutrient to microbiome and microRNA
- Comparison of Maternal Attachment and Maternal Role Confidence between Breast Milk in Sanitary Pack Feeding Infant's Mothers and Bottle Feeding Infant's Mothers of Low Birth Weight Infants in NICU
- Trans Fatty Acids of Breast Milk Lipids of Korean Women from Week 1 to 6 Months of Postpartum