Korean Circ J.
2021 Mar;51(3):267-278. 10.4070/kcj.2020.0345.
IgA Levels Are Associated with Coronary Artery Lesions in Kawasaki Disease
- Affiliations
-
- 1Asan Institute for Life Sciences, University of Ulsan College of Medicine, Seoul, Korea
- 2Department of Pediatrics, University of Ulsan College of Medicine, Asan Medical Center, Seoul, Korea
- 3Department of Pediatrics, Chung-Ang University Hospital, Seoul, Korea
- 4Department of Pediatrics, The Catholic University of Korea, Daejeon St. Mary's Hospital, Daejeon, Korea
- 5Department of Pediatrics, Kyung Hee University Hospital at Gangdong, Seoul, Korea
- 6Department of Pediatrics, Chungnam National University Hospital, Daejeon, Korea
- 7Department of Pediatrics, Seoul National University Children's Hospital, Seoul, Korea
- 8Department of Pediatrics, University of Ulsan, Gangneung Asan Hospital, Gangneung, Korea
- 9Department of Pediatrics, Inje University Paik Hospital, Busan, Korea
- 10Department of Pediatrics, Pusan National University Hospital, Busan, Korea
- 11Department of Pediatrics and Adolescent Medicine, Myongji Hospital, Goyang, Korea
- 12Department of Pediatrics, Korea University Guro Hospital, Seoul, Korea
- 13Department of Pediatrics, Ewha Womans University Hospital, Seoul, Korea
- 14Department of Pediatrics, Korea University Ansan Hospital, Ansan, Korea
- KMID: 2513525
- DOI: http://doi.org/10.4070/kcj.2020.0345
Abstract
- Background and Objectives
Kawasaki disease (KD) is an acute systemic vasculitis that affects the coronary arteries. Abnormal immune reactions are thought to contribute to disease pathogenesis. The effect of immunoglobulin (Ig) isotype (IgG, IgA, IgM, and IgE) on inflammatory data and clinical outcomes of patients with KD was examined.
Methods
Ig levels in 241 patients with KD were measured during the acute, subacute, convalescent, and normal phases of the disease.
Results
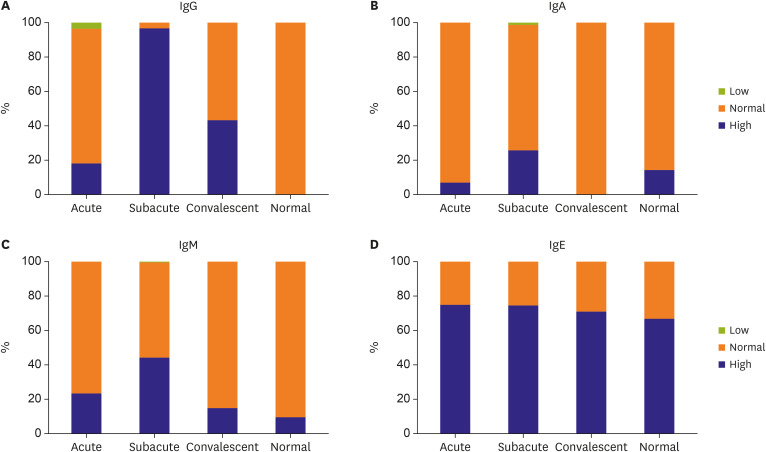
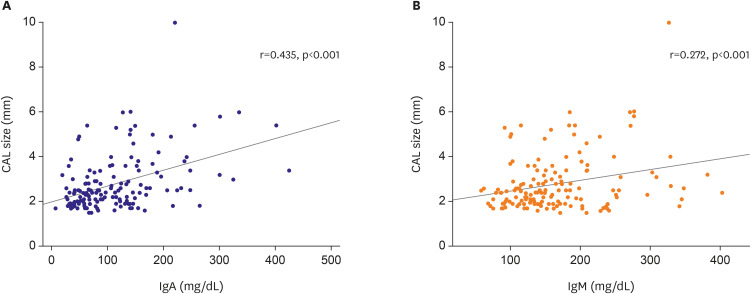
Compared with reference Ig values, IgG, IgA, and IgM levels were significantly higher in the subacute phase, while IgE levels were elevated in 73.9% (178/241) of patients with KD in all clinical phases. However, high IgE levels were not associated with clinical outcomes, including intravenous immunoglobulin unresponsiveness and coronary artery lesions (CALs). Significantly more CALs were observed in the high IgA group than in the normal IgA group (44.7% vs. 20.8%, respectively; p<0.01). In addition, IgA levels in the acute phase (p=0.038) were 2.2-fold higher, and those in the subacute phase were 1.7-fold higher (p <0.001), in the CAL group than in the non-CAL group. IgA concentrations increased along with the size of the coronary artery aneurysm (p <0.001). Furthermore, there was a strong correlation between IgA levels and CAL size (r=0.435, p<0.001), with a high odds ratio of 2.58 (p=0.022).
Conclusions
High IgA levels in patients with KD are prognostic for the risk of CALs.
Figure
Reference
-
1. Kawasaki T. Acute febrile mucocutaneous syndrome with lymphoid involvement with specific desquamation of the fingers and toes in children. Arerugi. 1967; 16:178–222. PMID: 6062087.2. Scuccimarri R. Kawasaki disease. Pediatr Clin North Am. 2012; 59:425–445. PMID: 22560578.3. Ding Y, Li G, Xiong LJ, et al. Profiles of responses of immunological factors to different subtypes of Kawasaki disease. BMC Musculoskelet Disord. 2015; 16:315. PMID: 26497060.4. Bayers S, Shulman ST, Paller AS. Kawasaki disease: part II. Complications and treatment. J Am Acad Dermatol. 2013; 69:513.e1–513.e8. PMID: 24034380.5. Newburger JW, Fulton DR. Kawasaki disease. Curr Opin Pediatr. 2004; 16:508–514. PMID: 15367843.6. Onouchi Y, Ozaki K, Burns JC, et al. A genome-wide association study identifies three new risk loci for Kawasaki disease. Nat Genet. 2012; 44:517–521. PMID: 22446962.7. Lee YC, Kuo HC, Chang JS, et al. Two new susceptibility loci for Kawasaki disease identified through genome-wide association analysis. Nat Genet. 2012; 44:522–525. PMID: 22446961.8. Khor CC, Davila S, Breunis WB, et al. Genome-wide association study identifies FCGR2A as a susceptibility locus for Kawasaki disease. Nat Genet. 2011; 43:1241–1246. PMID: 22081228.9. Kwon YC, Kim JJ, Yun SW, et al. BCL2L11 is associated with Kawasaki disease in intravenous immunoglobulin responder patients. Circ Genom Precis Med. 2018; 11:e002020. PMID: 29453247.10. Farh KK, Marson A, Zhu J, et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature. 2015; 518:337–343. PMID: 25363779.11. Onouchi Y. The genetics of Kawasaki disease. Int J Rheum Dis. 2018; 21:26–30. PMID: 29152908.12. Newburger JW, Takahashi M, Gerber MA, et al. Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki Disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation. 2004; 110:2747–2771. PMID: 15505111.13. Gregory GA, Andropoulos DB. Gregory's Pediatric Anesthesia, Fifth Edition. Hoboken (NJ): Blackwell Publishing Ltd.;2012. p. 1300–1314.14. Lindberg RE, Arroyave C. Levels of IgE in serum from normal children and allergic children as measured by an enzyme immunoassay. J Allergy Clin Immunol. 1986; 78:614–618. PMID: 3534049.15. Kim JJ, Yun SW, Yu JJ, et al. A genome-wide association analysis identifies NMNAT2 and HCP5 as susceptibility loci for Kawasaki disease. J Hum Genet. 2017; 62:1023–1029. PMID: 28855716.16. Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007; 81:559–575. PMID: 17701901.17. Kusakawa S, Heiner DC. Elevated levels of immunoglobulin E in the acute febrile mucocutaneous lymph node syndrome. Pediatr Res. 1976; 10:108–111. PMID: 1705.18. Koo CM, Choi SY, Kim DS, Kim KH. Relation between Kawasaki disease and immunoglobulin E. J Rheum Dis. 2013; 20:4–8.19. Liew WK, Lim CW, Tan TH, et al. The effect of Kawasaki disease on childhood allergies - a sibling control study. Pediatr Allergy Immunol. 2011; 22:488–493. PMID: 21443753.20. Kuo HC, Chang WC, Yang KD, et al. Kawasaki disease and subsequent risk of allergic diseases: a population-based matched cohort study. BMC Pediatr. 2013; 13:38. PMID: 23522327.21. Wei CC, Lin CL, Kao CH, et al. Increased risk of Kawasaki disease in children with common allergic diseases. Ann Epidemiol. 2014; 24:340–343. PMID: 24613197.22. Sawaji Y, Haneda N, Yamaguchi S, et al. Coronary risk factors in acute Kawasaki disease: correlation of serum immunoglobulin levels with coronary complications. Acta Paediatr Jpn. 1998; 40:218–225. PMID: 9695293.23. Yanagimoto K, Nomura Y, Masuda K, et al. Immunoglobulin G values before treatment are correlated with the responsiveness to initial intravenous immunoglobulin therapy for Kawasaki disease. Int Arch Allergy Immunol. 2014; 164:83–88. PMID: 24903098.24. Noval Rivas M, Wakita D, Franklin MK, et al. Intestinal permeability and IgA provoke immune vasculitis linked to cardiovascular inflammation. Immunity. 2019; 51:508–521.e6. PMID: 31471109.25. Rowley AH, Eckerley CA, Jäck HM, Shulman ST, Baker SC. IgA plasma cells in vascular tissue of patients with Kawasaki syndrome. J Immunol. 1997; 159:5946–5955. PMID: 9550392.26. Rowley AH, Shulman ST, Mask CA, et al. IgA plasma cell infiltration of proximal respiratory tract, pancreas, kidney, and coronary artery in acute Kawasaki disease. J Infect Dis. 2000; 182:1183–1191. PMID: 10979916.27. Jonsson S, Sveinbjornsson G, de Lapuente Portilla AL, et al. Identification of sequence variants influencing immunoglobulin levels. Nat Genet. 2017; 49:1182–1191. PMID: 28628107.28. Berndt SI, Camp NJ, Skibola CF, et al. Meta-analysis of genome-wide association studies discovers multiple loci for chronic lymphocytic leukemia. Nat Commun. 2016; 7:10933. PMID: 26956414.29. Wood CD, Veenstra H, Khasnis S, et al. MYC activation and BCL2L11 silencing by a tumour virus through the large-scale reconfiguration of enhancer-promoter hubs. Elife. 2016; 5:e18270. PMID: 27490482.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- CABG for an Adult with Coronary Disease due to Kawasaki Disease
- Percutaneous Transluminal Coronary Angioplasty for Coronary Artery Stenosis in an Adult Kawasaki Disease with Coronary Aneurysm : A Case Report and Review
- Laboratory Values in Patients with Kawasaki Disease after Intravenous Immunoglobulin: Comparison of Patients with Coronary Artery Lesions to those without Coronary Artery Lesions
- A Clinical Study of Atypical Kawasaki Disease: A Rate of Coronary Artery Involvement
- Changes in Coronary Perfusion after Occlusion of Coronary Arteries in Kawasaki Disease