Ann Surg Treat Res.
2021 Mar;100(3):127-136. 10.4174/astr.2021.100.3.127.
Selective inhibition of V600E-mutant BRAF gene induces apoptosis in thyroid carcinoma cell lines
- Affiliations
-
- 1Department of Surgery, Konkuk University School of Medicine, Seoul, Korea
- 2Department of Surgery, Konkuk University Medical Center, Seoul, Korea
- 3Research Institute of Medical Science, Konkuk University School of Medicine, Seoul, Korea
- 4Institute of Botany and Molecular Genetics, RWTH, Aachen University, Aachen, Germany
- 5Research Centers for Cellular Homeostasis, Ewha Womans University, Seoul, Korea
- 6Cancer Research Institute, Seoul National University College of Medicine, Seoul, Korea
- 7Department of Surgery, School of Medicine, Kyung Hee University, Seoul, Korea
- 8Thyroid Clinic, St. Peter’s Hospital, Seoul, Korea
- KMID: 2513193
- DOI: http://doi.org/10.4174/astr.2021.100.3.127
Abstract
- Purpose
Papillary thyroid cancer (PTC) has a high incidence of BRAF V600E mutation. The purpose of this study was to evaluate the potential relationship between thyroiditis and BRAF V600E mutation status in patients with PTC. We investigated how a selective inhibitor of BRAF V600E PLX4032 affects the proliferation and inflammatory cytokine levels of thyroid cancer.
Methods
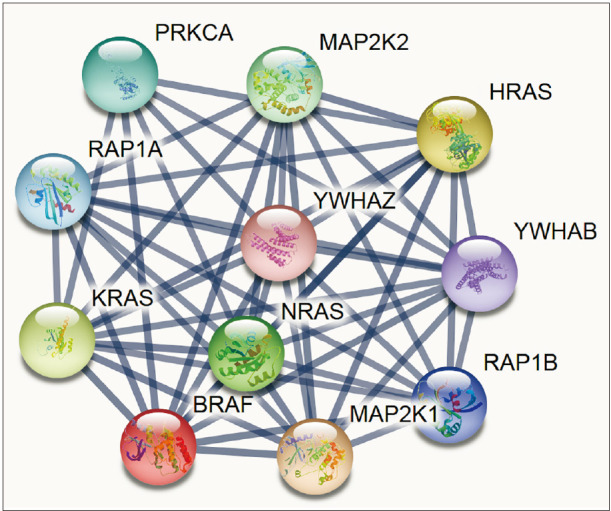
Two thyroid cancer cell lines TPC1 and 8505C were treated with PLX4032, an analysis was done on cell growth, cell cycle, the degree of apoptosis, and levels of inflammatory cytokines. To identify the functional links of BRAF, we used the STRING database.
Results
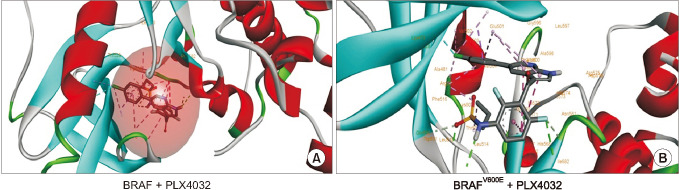
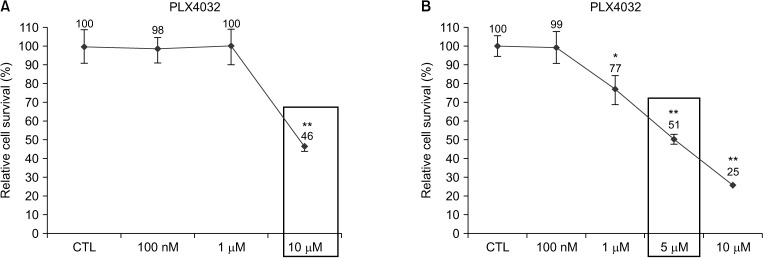
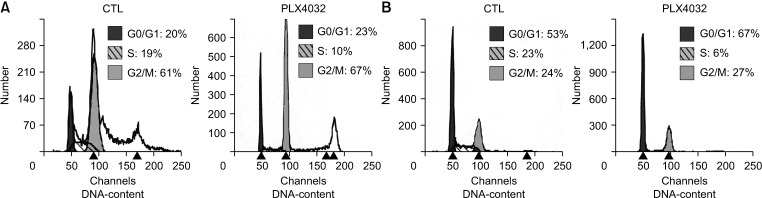
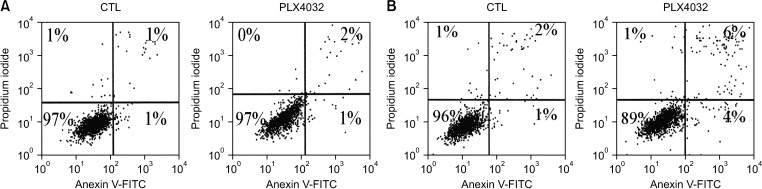
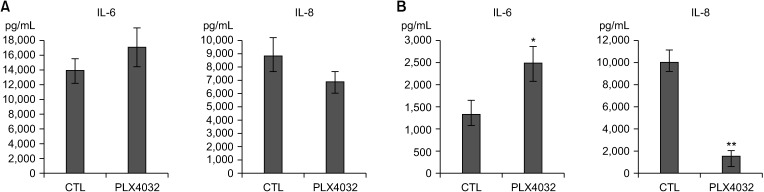
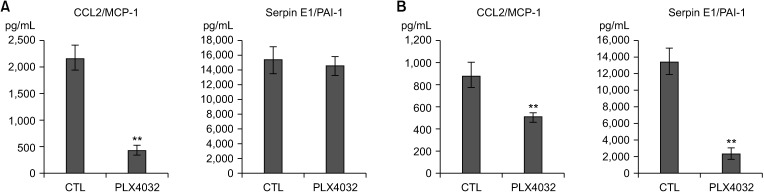
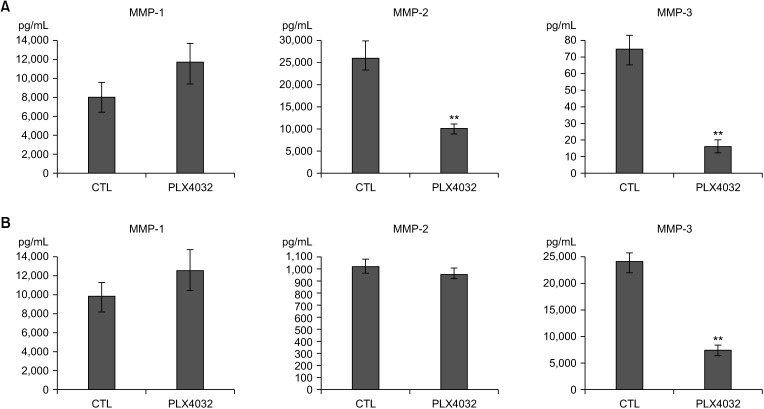
Docking results illustrated PLX4032 blocked the kinase activity by exclusively binding on the serine/threonine kinase domain. STRING results indicated BRAF is functionally linked to mitogen-activated protein kinase. Both cell lines showed a dose-dependent reduction in growth rate but had a different half maximal inhibitory concentration value for PLX4032. The reaction to PLX4032 was more sensitive in the 8505C cells than in the TPC1 cells. PLX4032 induced a G2/ M phase arrest in the TPC1 cells and G0/G1 in the 8505C cells. PLX4032 induced apoptosis only in the 8505C cells. With PLX4032, the TPC1 cells showed decreased levels of vascular endothelial growth factor, granulocyte-macrophage colonystimulating factor, chemokine (C-C motif) ligand 2/monocyte chemoattractant protein 1, whereas the 8505C cells showed significantly decreased levels of IL-8, serpin E1/plasminogen activator inhibitor-1, and matrix metalloproteinase (MMP)-3.
Conclusion
PLX4032 was cytotoxic in both TPC1 and 8505C cells and induced apoptosis. In the 8505C cells, inflammatory cytokines such as IL-8 and MMP-3 were down-regulated. These findings suggest the possibility that the BRAF V600E mutation needs to target inflammatory signaling pathways in the treatment of thyroid cancer.
Keyword
Figure
Reference
-
1. Won YJ, Sung J, Jung KW, Kong HJ, Park S, Shin HR, et al. Nationwide cancer incidence in Korea, 2003–2005. Cancer Res Treat. 2009; 41:122–131. PMID: 19809561.
Article2. Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007; 57:43–66. PMID: 17237035.
Article3. Larson SD, Jackson LN, Riall TS, Uchida T, Thomas RP, Qiu S, et al. Increased incidence of well-differentiated thyroid cancer associated with Hashimoto thyroiditis and the role of the PI3k/Akt pathway. J Am Coll Surg. 2007; 204:764–773. PMID: 17481480.
Article4. Zhang L, Li H, Ji QH, Zhu YX, Wang ZY, Wang Y, et al. The clinical features of papillary thyroid cancer in Hashimoto's thyroiditis patients from an area with a high prevalence of Hashimoto's disease. BMC Cancer. 2012; 12:610. PMID: 23256514.
Article5. Teng W, Shan Z, Teng X, Guan H, Li Y, Teng D, et al. Effect of iodine intake on thyroid diseases in China. N Engl J Med. 2006; 354:2783–2793. PMID: 16807415.
Article6. Kobawala TP, Trivedi TI, Gajjar KK, Patel DH, Patel GH, Ghosh NR. Significance of interleukin-6 in papillary thyroid carcinoma. J Thyroid Res. 2016; 2016:6178921. PMID: 27034885.
Article7. Kobawala TP, Patel GH, Gajjar DR, Patel KN, Thakor PB, Parekh UB, et al. Clinical utility of serum interleukin-8 and interferon-alpha in thyroid diseases. J Thyroid Res. 2011; 2011:270149. PMID: 21461397.
Article8. Stetler-Stevenson WG. The role of matrix metalloproteinases in tumor invasion, metastasis, and angiogenesis. Surg Oncol Clin N Am. 2001; 10:383–392. PMID: 11382593.
Article9. Kebebew E, Weng J, Bauer J, Ranvier G, Clark OH, Duh QY, et al. The prevalence and prognostic value of BRAF mutation in thyroid cancer. Ann Surg. 2007; 246:466–470. PMID: 17717450.
Article10. Nucera C, Lawler J, Parangi S. BRAF(V600E) and microenvironment in thyroid cancer: a functional link to drive cancer progression. Cancer Res. 2011; 71:2417–2422. PMID: 21447745.11. Sala E, Mologni L, Truffa S, Gaetano C, Bollag GE, Gambacorti-Passerini C. BRAF silencing by short hairpin RNA or chemical blockade by PLX4032 leads to different responses in melanoma and thyroid carcinoma cells. Mol Cancer Res. 2008; 6:751–759. PMID: 18458053.
Article12. Meireles AM, Preto A, Rocha AS, Rebocho AP, Máximo V, Pereira-Castro I, et al. Molecular and genotypic characterization of human thyroid follicular cell carcinoma-derived cell lines. Thyroid. 2007; 17:707–715. PMID: 17725429.
Article13. Koh CS, Ku JL, Park SY, Kim KH, Choi JS, Kim IJ, et al. Establishment and characterization of cell lines from three human thyroid carcinomas: responses to all-trans-retinoic acid and mutations in the BRAF gene. Mol Cell Endocrinol. 2007; 264:118–127. PMID: 17134824.
Article14. Nadeau V, Guillemette S, Bélanger LF, Jacob O, Roy S, Charron J. Map2k1 and Map2k2 genes contribute to the normal development of syncytiotrophoblasts during placentation. Development. 2009; 136:1363–1374. PMID: 19304888.15. Dubois T, Rommel C, Howell S, Steinhussen U, Soneji Y, Morrice N, et al. 14-3-3 is phosphorylated by casein kinase I on residue 233. Phosphorylation at this site in vivo regulates Raf/14-3-3 interaction. J Biol Chem. 1997; 272:28882–28888. PMID: 9360956.16. Halaban R, Zhang W, Bacchiocchi A, Cheng E, Parisi F, Ariyan S, et al. PLX4032, a selective BRAF(V600E) kinase inhibitor, activates the ERK pathway and enhances cell migration and proliferation of BRAF melanoma cells. Pigment Cell Melanoma Res. 2010; 23:190–200. PMID: 20149136.17. Emery CM, Vijayendran KG, Zipser MC, Sawyer AM, Niu L, Kim JJ, et al. MEK1 mutations confer resistance to MEK and B-RAF inhibition. Proc Natl Acad Sci U S A. 2009; 106:20411–20416. PMID: 19915144.
Article18. Salerno P, De Falco V, Tamburrino A, Nappi TC, Vecchio G, Schweppe RE, et al. Cytostatic activity of adenosine triphosphate-competitive kinase inhibitors in BRAF mutant thyroid carcinoma cells. J Clin Endocrinol Metab. 2010; 95:450–455. PMID: 19880792.
Article19. Wang L, Yi T, Zhang W, Pardoll DM, Yu H. IL-17 enhances tumor development in carcinogen-induced skin cancer. Cancer Res. 2010; 70:10112–10120. PMID: 21159633.
Article20. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008; 454:436–444. PMID: 18650914.
Article21. Pikarsky E, Porat RM, Stein I, Abramovitch R, Amit S, Kasem S, et al. NF-kappaB functions as a tumour promoter in inflammation-associated cancer. Nature. 2004; 431:461–466. PMID: 15329734.22. Muzza M, Degl'Innocenti D, Colombo C, Perrino M, Ravasi E, Rossi S, et al. The tight relationship between papillary thyroid cancer, autoimmunity and inflammation: clinical and molecular studies. Clin Endocrinol (Oxf). 2010; 72:702–708. PMID: 20447069.
Article23. Lumachi F, Basso SM, Orlando R. Cytokines, thyroid diseases and thyroid cancer. Cytokine. 2010; 50:229–233. PMID: 20381375.
Article24. Reynolds JV, Donohoe CL, Doyle SL. Diet, obesity and cancer. Ir J Med Sci. 2011; 180:521–527. PMID: 21174166.
Article25. Crawford S, Belajic D, Wei J, Riley JP, Dunford PJ, Bembenek S, et al. A novel B-RAF inhibitor blocks interleukin-8 (IL-8) synthesis in human melanoma xenografts, revealing IL-8 as a potential pharmacodynamic biomarker. Mol Cancer Ther. 2008; 7:492–499. PMID: 18347137.
Article26. Linkov F, Ferris RL, Yurkovetsky Z, Marrangoni A, Velikokhatnaya L, Gooding W, et al. Multiplex analysis of cytokines as biomarkers that differentiate benign and malignant thyroid diseases. Proteomics Clin Appl. 2008; 2:1575–1585. PMID: 19234619.
Article27. Kim S, Park YW, Schiff BA, Doan DD, Yazici Y, Jasser SA, et al. An orthotopic model of anaplastic thyroid carcinoma in athymic nude mice. Clin Cancer Res. 2005; 11:1713–1721. PMID: 15755992.
Article28. Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, et al. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009; 69:1302–1313. PMID: 19190339.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Induction of the BRAFV600E Mutation in Thyroid Cells Leads to Frequent Hypermethylation
- BRAFV600E Mutation Enhances Estrogen-Induced Metastatic Potential of Thyroid Cancer by Regulating the Expression of Estrogen Receptors
- Induction of Resistance to BRAF Inhibitor Is Associated with the Inability of Spry2 to Inhibit BRAF-V600E Activity in BRAF Mutant Cells
- Association of BRAF(V600E) Mutation with Poor Clinical Prognostic Factors and Ultrasonographic Findings in Cases of Papillary Thyroid Carcinoma
- Clinical Implication of BRAF Mutation in Thyroid Cancer