Int J Stem Cells.
2021 Feb;14(1):47-57. 10.15283/ijsc20025.
First Year Results of Suprachoroidal Adipose Tissue Derived Mesenchymal Stem Cell Implantation in Degenerative Macular Diseases
- Affiliations
-
- 1Ophthalmology Department, Kayseri Acibadem Hospital, Kayseri, Turkey
- 2Genome and Stem Cell Center, Kayseri, Turkey
- 3Ophthalmology Department, Erciyes University, Kayseri, Turkey
- KMID: 2513082
- DOI: http://doi.org/10.15283/ijsc20025
Abstract
- Background and Objectives
This study shows the clinical data of 1-year follow-up of 8 patients with degenerative macular diseases who received suprachoroidal adipose tissue derived mesenchymal stem cell (ADMSC) implantation.
Methods and Results
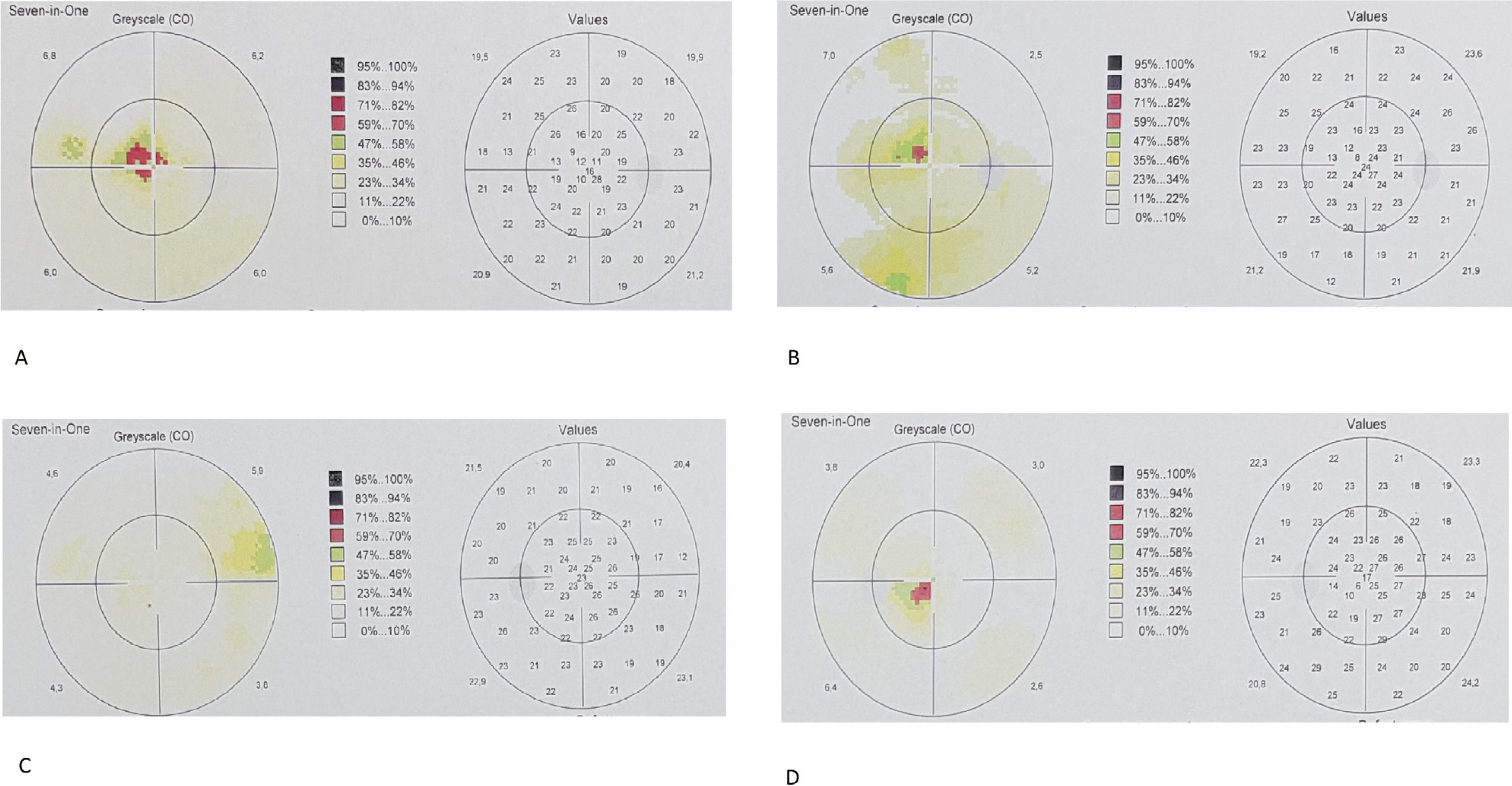
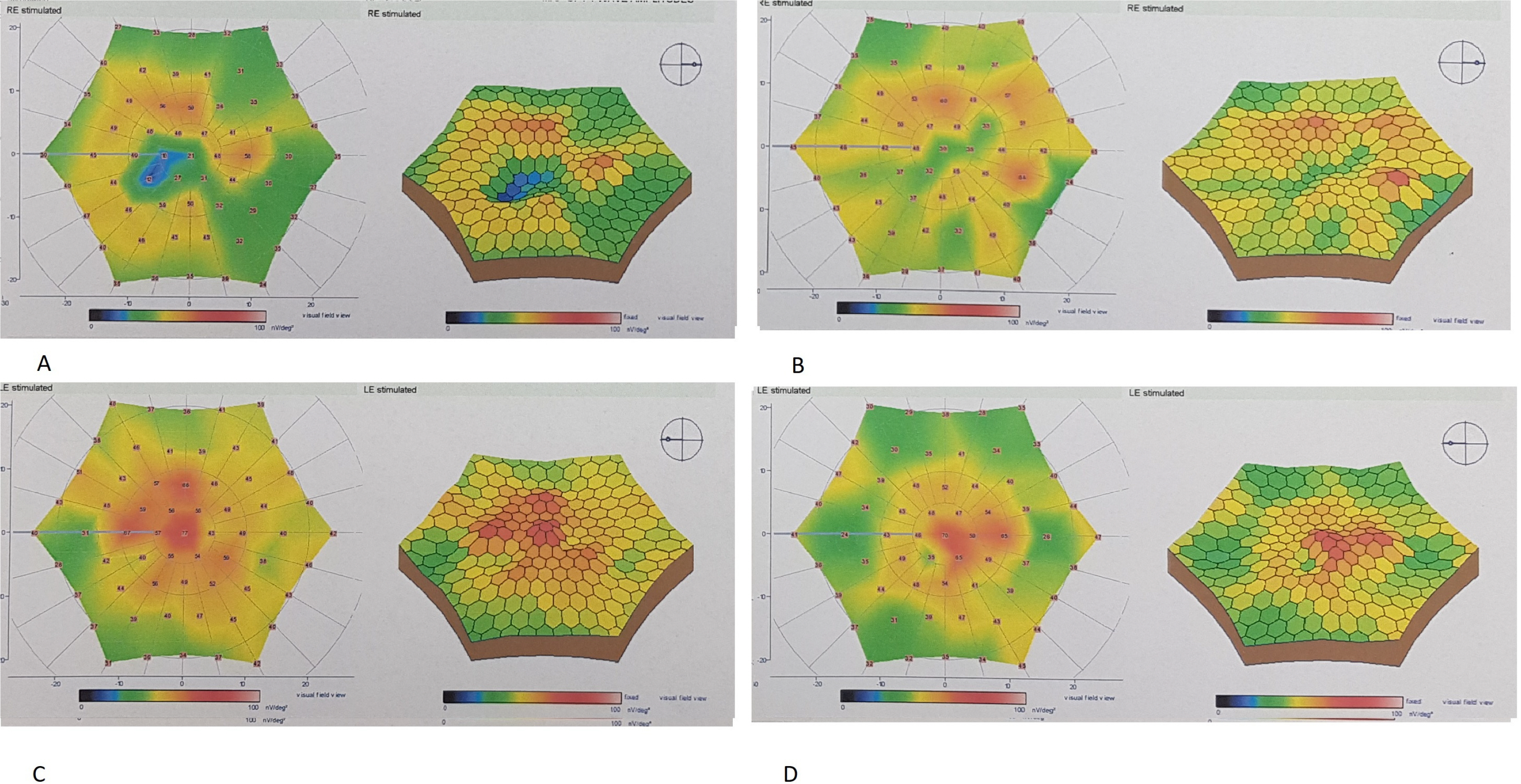
This prospective, single-center, phase 1/2 study enrolled 8 eyes of 8 patients with degenerative macular diseases of various reasons who underwent suprachoroidal implantation of ADMSCs. All patients had severe visual field defects and severe visual loss. All patients had defective multifocal electroretinography (mf ERG). The worse eye of the patient was selected for the operation. Patients were evaluated on the first day, first month, sixth month and at 1 year postoperatively. Best corrected visual acuity (BCVA), anterior segment and fundus examination, color photography, optical coherence tomography (OCT) and visual field (VF) examination were carried out at each visit. Fundus fluorescein angiography (FFA) and mfERG recordings were performed at the end of the sixth months. All 8 patients completed the 1 year follow-up. None of them had any systemic or ocular complications. Seven of the patients experienced visual acuity improvement, visual field improvement and improvement in the mfERG recordings. We found choroidal thickening in OCT of the four treated eyes.
Conclusions
Even though the sample size is small, stem cell treatment with suprachoroidal implantation of ADMSCs seems to be safe and the improvements were encouraging. To optimize the cell delivery technique and to evaluate the effects of this therapy on visual acuity and the quality of life of these patients, future studies with larger number of cases will be necessary.
Figure
Reference
-
References
1. Öner A. 2018; Stem cell treatment in retinal diseases: recent developments. Turk J Ophthalmol. 48:33–38. DOI: 10.4274/tjo.89972. PMID: 29576896. PMCID: PMC5854857.
Article2. Kahraman NS, Öner A. 2019; Stem cell treatment in degenerative retinal and optic nerve diseases. Trak Univ J Nat Sci. 20:11–16.3. Mead B, Berry M, Logan A, Scott RA, Leadbeater W, Scheven BA. 2015; Stem cell treatment of degenerative eye disease. Stem Cell Res. 14:243–257. DOI: 10.1016/j.scr.2015.02.003. PMID: 25752437. PMCID: PMC4434205.
Article4. Kalbermatten DF, Schaakxs D, Kingham PJ, Wiberg M. 2011; Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 344:251–260. DOI: 10.1007/s00441-011-1142-5. PMID: 21400216.
Article5. Mead B, Logan A, Berry M, Leadbeater W, Scheven BA. 2014; Paracrine-mediated neuroprotection and neuritogenesis of axotomised retinal ganglion cells by human dental pulp stem cells: comparison with human bone marrow and adipose-derived mesenchymal stem cells. PLoS One. 9:e109305. DOI: 10.1371/journal.pone.0109305. PMID: 25290916. PMCID: PMC4188599.
Article6. Tang Z, Zhang Y, Wang Y, Zhang D, Shen B, Luo M, Gu P. 2017; Progress of stem/progenitor cell-based therapy for retinal degeneration. J Transl Med. 15:99. DOI: 10.1186/s12967-017-1183-y. PMID: 28486987. PMCID: PMC5424366.
Article7. Khan M, Agarwal K, Loutfi M, Kamal A. 2014; Present and possible therapies for age-related macular degeneration. ISRN Ophthalmol. 2014:608390. DOI: 10.1155/2014/608390. PMID: 25097787. PMCID: PMC4009180.
Article8. Fujinami K, Lois N, Davidson AE, Mackay DS, Hogg CR, Stone EM, Tsunoda K, Tsubota K, Bunce C, Robson AG, Moore AT, Webster AR, Holder GE, Michaelides M. 2013; A longitudinal study of stargardt disease: clinical and electrophysiologic assessment, progression, and genotype correla-tions. Am J Ophthalmol. 155:1075–1088.e13. DOI: 10.1016/j.ajo.2013.01.018. PMID: 23499370.
Article9. Hood DC, Bach M, Brigell M, Keating D, Kondo M, Lyons JS, Marmor MF, McCulloch DL, Palmowski-Wolfe AM. 2012; ISCEV standard for clinical multifocal electroretinography (mfERG) (2011 edition). Doc Ophthalmol. 124:1–13. DOI: 10.1007/s10633-011-9296-8. PMID: 22038576. PMCID: PMC4466109.
Article10. Limoli PG, Vingolo EM, Morales MU, Nebbioso M, Limoli C. 2014; Preliminary study on electrophysiological changes after cellular autograft in age-related macular degeneration. Medicine (Baltimore). 93:e355. DOI: 10.1097/MD.0000000000000355. PMID: 25546695. PMCID: PMC4602619.
Article11. Oner A, Gonen ZB, Sinim N, Cetin M, Ozkul Y. 2016; Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Res Ther. 7:178. DOI: 10.1186/s13287-016-0432-y. PMID: 27906070. PMCID: PMC5134260.
Article12. Kahraman NS, Sevim DG, Öner A. 2019; Cross-validation of the Turkish version of the 28-item impact of vision impairment profile test. Open J Ophthalmol. 9:194–202. DOI: 10.4236/ojoph.2019.94021.
Article13. Oner A, Gonen ZB, Sevim DG, Sinim N, Cetin M, Ozkul Y. 2019; First-year results of subretinal mesenchymal stem cell implantation in severe retinitis pigmentosa. J Stem Cell Res Ther. 9:454.14. Limoli PG, Vingolo EM, Limoli C, Scalinci SZ, Nebbioso M. 2018; Regenerative therapy by suprachoroidal cell autograft in dry age-related macular degeneration: preliminary in vivo report. J Vis Exp. (132):56469. DOI: 10.3791/56469. PMID: 29553543. PMCID: PMC5912396.
Article15. Habot-Wilner Z, Noronha G, Wykoff CC. 2019; Suprachoroidally injected pharmacological agents for the treatment of chorio-retinal diseases: a targeted approach. Acta Ophthalmol. 97:460–472. DOI: 10.1111/aos.14042. PMID: 30702218.
Article16. Oner A, Gonen ZB, Sevim DG, Smim Kahraman N, Unlu M. 2018; Suprachoroidal adipose tissue-derived mesenchymal stem cell implantation in patients with dry-type age-related macular degeneration and Stargardt's macular dystrophy: 6-month follow-up results of a phase 2 study. Cell Reprogram. 20:329–336. DOI: 10.1089/cell.2018.0045. PMID: 31251672.
Article17. Thanos C, Emerich D. 2005; Delivery of neurotrophic factors and therapeutic proteins for retinal diseases. Expert Opin Biol Ther. 5:1443–1452. DOI: 10.1517/14712598.5.11.1443. PMID: 16255648.
Article18. Schäffler A, Büchler C. 2007; Concise review: adipose tissue-derived stromal cells--basic and clinical implications for novel cell-based therapies. Stem Cells. 25:818–827. DOI: 10.1634/stemcells.2006-0589. PMID: 17420225.
Article19. Tischler M. 2002; Platelet rich plasma. The use of autologous growth factors to enhance bone and soft tissue grafts. N Y State Dent J. 68:22–24. PMID: 11989332.20. Kahraman NS, Oner A. 2020; Subtenon injection of autologous platelet-rich plasma in retinitis pigmentosa: is it a new therapeutic option? Open J Ophthalmol. 10:77–88. DOI: 10.4236/ojoph.2020.101010.
Article21. Kamao H, Mandai M, Okamoto S, Sakai N, Suga A, Sugita S, Kiryu J, Takahashi M. 2014; Characterization of human induced pluripotent stem cell-derived retinal pigment epithelium cell sheets aiming for clinical application. Stem Cell Reports. 2:205–218. DOI: 10.1016/j.stemcr.2013.12.007. PMID: 24527394. PMCID: PMC3923225.
Article22. Dang Y, Zhang C, Zhu Y. 2015; Stem cell therapies for age-related macular degeneration: the past, present, and future. Clin Interv Aging. 10:255–264. DOI: 10.2147/CIA.S73705. PMID: 25609937. PMCID: PMC4298283.
Article23. Limoli PG, Limoli C, Vingolo EM, Scalinci SZ, Nebbioso M. 2016; Cell surgery and growth factors in dry age-related macular degeneration: visual prognosis and morphological study. Oncotarget. 7:46913–46923. DOI: 10.18632/oncotarget.10442. PMID: 27391437. PMCID: PMC5216913.
Article24. Limoli PG, Vingolo EM, Limoli C, Nebbioso M. 2019; Stem cell surgery and growth factors in retinitis pigmentosa patients: pilot study after literature review. Biomedicines. 7:94. DOI: 10.3390/biomedicines7040094. PMID: 31801246. PMCID: PMC6966474.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Concise Review: Differentiation of Human Adult Stem Cells Into Hepatocyte-like Cells In vitro
- Adipose-derived stem cells: characterization and clinical application
- Adipose Tissue - Adequate, Accessible Regenerative Material
- Adipose Tissue Derived Mesenchymal Stem Cells
- L-Theanine-Treated Adipose-Derived Mesenchymal Stem Cells Alleviate the Cytotoxicity Induced by N-Nitrosodiethylamine in Liver