Ann Lab Med.
2021 Jul;41(4):386-393. 10.3343/alm.2021.41.4.386.
Ten-Year Prevalence Trends of Phenotypically Identified Community-Associated Methicillin-Resistant Staphylococcus aureus Strains in Clinical Specimens
- Affiliations
-
- 1Chung-Ang University College of Medicine, Chung-Ang University Hospital, Seoul, Korea
- 2Division of Infectious Diseases, Department of Internal Medicine, Chung-Ang University Hospital, Seoul, Korea
- 3Department of Laboratory Medicine, Chung-Ang University Hospital, Seoul, Korea
- KMID: 2512718
- DOI: http://doi.org/10.3343/alm.2021.41.4.386
Abstract
- Background
Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA) strains were first detected in hospitals in Korea between the late 2000s and early 2010s. However, there is limited information regarding the prevalence of CA-MRSA strains among hospital isolates and their phenotypic changes over the last decade. We investigated the prevalence trend of CA-MRSA strains isolated from different clinical specimens and their phenotypic changes between September 2009 and September 2019.
Methods
CA-MRSA strains were phenotypically identified by confirming their resistance to penicillin (PCN) and oxacillin (OXA) and evaluating their susceptibility to trimethoprimsulfamethoxazole, rifampin, fusidic acid, tetracycline, and at least one of the following four antimicrobials: clindamycin (CLI), erythromycin (ERY), ciprofloxacin (CIP), and gentamicin (GEN). A CA-MRSA strain that exhibited resistance to ERY, CLI, CIP, or GEN was classified as having resistance pattern I, II, III, or IV, respectively, regardless of its resistance to other antimicrobial agents.
Results
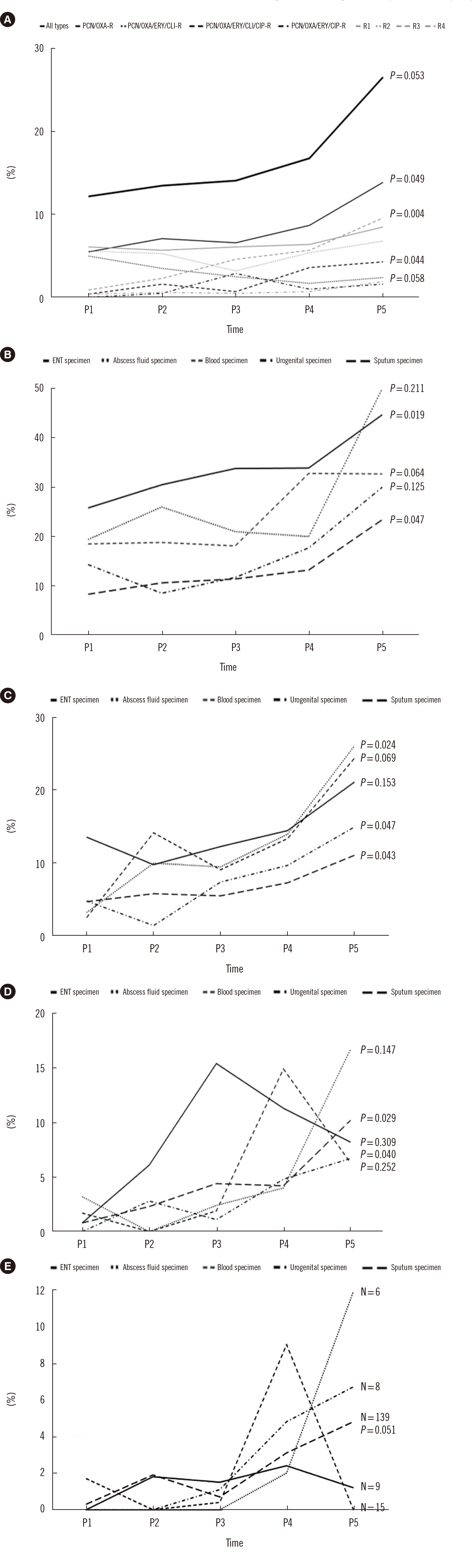
Of the 8,278 MRSA isolates identified in specimens obtained two days after admission, 1,385 (16.73%) were CA-MRSA strains. The prevalence of CA-MRSA strains increased from 12.2% to 26.6% (3.21% per period, P = 0.05). Resistance type analysis revealed an increasing trend in the prevalence of PCN/OXA-resistant (1.84%; P = 0.049) and PCN/OXA/ERY/CLI/CIP-resistant (0.98%; P = 0.04) CA-MRSA strains and in resistance pattern III strains (2.08%; P = 0.004).
Conclusions
The prevalence of CA-MRSA strains in Korea has increased significantly over the last decade, and CA-MRSA strains have gained phenotypic diversity beyond PCN/OXAresistance, including antimicrobial resistance to non-β-lactams, especially CIP.
Keyword
Figure
Cited by 2 articles
-
In-vitro Activity of Delpazolid and Comparator Agents Against Methicillin-resistantStaphylococcus aureus Involved in Bloodstream Infection
Jeong Su Park, Yun Jung Choi, Kyungmi Kwon, Seong Jin Choi, Song Mi Moon, Kyoung-Ho Song, Eu Suk Kim, Kyoung Un Park, Hong Bin Kim
Ann Lab Med. 2023;43(4):389-391. doi: 10.3343/alm.2023.43.4.389.European Committee on Antimicrobial Susceptibility Testing-Recommended Rapid Antimicrobial Susceptibility Testing of
Escherichia coli ,Klebsiella pneumoniae , andStaphylococcus aureus From Positive Blood Culture Bottles
Jong-Min Park, Mijung Kwon, Ki Ho Hong, Hyukmin Lee, Dongeun Yong
Ann Lab Med. 2023;43(5):443-450. doi: 10.3343/alm.2023.43.5.443.
Reference
-
1. Stryjewski ME, Corey GR. 2014; Methicillin-resistant Staphylococcus aureus: an evolving pathogen. Clin Infect Dis. 58:S10–9. DOI: 10.1093/cid/cit613. PMID: 24343827.2. David MZ, Daum RS. 2010; Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 23:616–87. DOI: 10.1128/CMR.00081-09. PMID: 20610826. PMCID: PMC2901661.3. Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, et al. 2007; A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 60:1108–14. DOI: 10.1093/jac/dkm309. PMID: 17884831.4. Seybold U, Kourbatova EV, Johnson JG, Halvosa SJ, Wang YF, King MD, et al. 2006; Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of healthcare-associated bloodstream infections. Clin Infect Dis. 42:647–56. DOI: 10.1086/499815. PMID: 16447110.5. Souli M, Ruffin F, Choi SH, Park LP, Gao S, Lent NC, et al. 2019; Changing characteristics of Staphylococcus aureus bacteremia: results from a 21-year, prospective, longitudinal study. Clin Infect Dis. 69:1868–77. DOI: 10.1093/cid/ciz112. PMID: 31001618. PMCID: PMC6853684.6. Joo EJ, Chung DR, Ha YE, Park SY, Kang SJ, Kim SH, et al. 2012; Community-associated Panton-Valentine leukocidin-negative meticillin-resistant Staphylococcus aureus clone (ST72-MRSA-IV) causing healthcare-associated pneumonia and surgical site infection in Korea. J Hosp Infect. 81:149–55. DOI: 10.1016/j.jhin.2012.04.018. PMID: 22652522.7. Park SY, Chung DR, Yoo JR, Baek JY, Kim SH, Ha YE, et al. 2016; Sequence type 72 community-associated meticillin-resistant Staphylococcus aureus emerged as a predominant clone of nasal colonization in newly admitted patients. J Hosp Infect. 93:386–9. DOI: 10.1016/j.jhin.2015.12.008. PMID: 26874934.8. Joo EJ, Chung DR, Kim SH, Baek JY, Lee NY, Cho SY, et al. 2017; Emergence of community-genotype methicillin-resistant Staphylococcus aureus in Korean hospitals: clinical characteristics of nosocomial infections by community-genotype strain. Infect Chemother. 49:109–16. DOI: 10.3947/ic.2017.49.2.109. PMID: 28608660. PMCID: PMC5500265.9. Joo EJ, Chung DR, Ha YE, Park SY, Kim HA, Lim MH, et al. 2012; Clinical predictors of community-genotype ST72-methicillin-resistant Staphylococcus aureus-SCCmec type IV in patients with community-onset S. aureus infection. J Antimicrob Chemother. 67:1755–9. DOI: 10.1093/jac/dks120. PMID: 22514264.10. Shin E, Hong H, Park J, Oh Y, Jung J, Lee Y. 2016; Characterization of Staphylococcus aureus faecal isolates associated with food-borne disease in Korea. J Appl Microbiol. 121:277–86. DOI: 10.1111/jam.13133. PMID: 26991816.11. Lee J, Sung JY, Kim YM, Oh CE, Kim HB, Choi EH, et al. 2011; Molecular characterization of methicillin-resistant Staphylococcus aureus obtained from the anterior nares of healthy Korean children attending daycare centers. Int J Infect Dis. 15:e558–63. DOI: 10.1016/j.ijid.2011.04.010. PMID: 21664849.12. Ko KS, Lee JY, Baek JY, Peck KR, Rhee JY, Kwon KT, et al. 2008; Characterization of Staphylococcus aureus nasal carriage from children attending an outpatient clinic in Seoul, Korea. Microb Drug Resist. 14:37–44. DOI: 10.1089/mdr.2008.0776. PMID: 18346010.13. Sung JY, Lee J, Choi EH, Lee HJ. 2012; Changes in molecular epidemiology of community-associated and health care-associated methicillin-resistant Staphylococcus aureus in Korean children. Diagn Microbiol Infect Dis. 74:28–33. DOI: 10.1016/j.diagmicrobio.2012.05.018. PMID: 22722011.14. Kim ES, Song JS, Lee HJ, Choe PG, Park KH, Cho JH, et al. 2008; A survey of community-associated methicillin-resistant Staphylococcus aureus in Korea. J Antimicrob Chemother. 62:214. DOI: 10.1093/jac/dkn171.15. Peck KR, Baek JY, Song JH, Ko KS. 2009; Comparison of genotypes and enterotoxin genes between Staphylococcus aureus isolates from blood and nasal colonizers in a Korean hospital. J Korean Med Sci. 24:585–91. DOI: 10.3346/jkms.2009.24.4.585. PMID: 19654937. PMCID: PMC2719184.16. Bae IG, Kim JS, Kim S, Heo ST, Chang C, Lee EY. 2010; Genetic correlation of community-associated methicillin-resistant Staphylococcus aureus strains from carriers and from patients with clinical infection in one region of Korea. J Korean Med Sci. 25:197–202. DOI: 10.3346/jkms.2010.25.2.197. PMID: 20119570. PMCID: PMC2811284.17. Moon SY, Lee HJ, Lee MS. 2010; Molecular characteristics of methicillin-resistant Staphylococcus aureus blood isolates: clonal spread of staphylococcal cassette chromosome mec type IVA between the community and the hospital. Microb Drug Resist. 16:217–22. DOI: 10.1089/mdr.2010.0010. PMID: 20735176.18. Kwon JC, Kim SH, Park SH, Choi SM, Lee DG, Choi JH, et al. 2011; Molecular epidemiologic analysis of methicillin-resistant Staphylococcus aureus isolates from bacteremia and nasal colonization at 10 intensive care units: multicenter prospective study in Korea. J Korean Med Sci. 26:604–11. DOI: 10.3346/jkms.2011.26.5.604. PMID: 21532849. PMCID: PMC3082110.19. Park KH, Chong YP, Kim SH, Lee SO, Choi SH, Lee MS, et al. 2015; Community-associated MRSA strain ST72-SCCmecIV causing bloodstream infections: clinical outcomes and bacterial virulence factors. J Antimicrob Chemother. 70:1185–92. DOI: 10.1093/jac/dku475.20. Bae E, Kim CK, Jang JH, Sung H, Choi Y, Kim MN. 2019; Impact of community-onset methicillin-resistant Staphylococcus aureus on Staphylococcus aureus bacteremia in a central Korea Veterans Health Service hospital. Ann Lab Med. 39:158–66. DOI: 10.3343/alm.2019.39.2.158. PMID: 30430778. PMCID: PMC6240515.21. Park SH, Park C, Yoo JH, Choi SM, Choi JH, Shin HH, et al. 2009; Emergence of community-associated methicillin-resistant Staphylococcus aureus strains as a cause of healthcare-associated bloodstream infections in Korea. Infect Control Hosp Epidemiol. 30:146–55. DOI: 10.1086/593953. PMID: 19128184.22. Choi SH, Lee J, Jung J, Kim ES, Kim MJ, Chong YP, et al. A longitudinal study of adult patients with Staphylococcus aureus bacteremia over 11 years in South Korea. J Korean Med Sci. [in press].23. CLSI. 2015. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100-S25. 25th ed. Clinical and Laboratory Standards Institute;Wayne, PA:24. Yoo IY, Kang OK, Shim HJ, Huh HJ, Lee NY. 2020; Linezolid resistance in methicillin-resistant Staphylococcus aureus in Korea: high rate of false resistance to linezolid by the VITEK 2 system. Ann Lab Med. 40:57–62. DOI: 10.3343/alm.2020.40.1.57. PMID: 31432640. PMCID: PMC6713661.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Detection of Multidrug Resistant Patterns and Associated - genes of Methicillin Resistant Staphylococcus aureus ( MRSA ) Isolated from Clinical Specimens
- A case of multiple furunculosis caused by methicillin-resistant staphylococcs aureus
- A Survey for Methicillin - Resistant Staphylococcus Aureus
- Community-associated methicillin-resistant Staphylococcus aureus (CA-MRSA)
- Molecular Epidemiologic Methods Used in the Analysis of Methicillin-Resistant staphylococcus aureus