Recent Trends in Creatinine Assays in Korea: LongTerm Accuracy-Based Proficiency Testing Survey Data by the Korean Association of External Quality Assessment Service (2011–2019)
- Affiliations
-
- 1Department of Laboratory Medicine, Ewha Womans University College of Medicine, Seoul, Korea
- 2Department of Laboratory Medicine, Hallym University Dongtan Sacred Heart Hospital, Hallym University College of Medicine, Hwaseong, Korea
- 33 Department of Laboratory Medicine, Seoul National University Bundang Hospital and College of Medicine, Seongnam, Korea
- 4Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 5Department of Laboratory Medicine, Konkuk University School of Medicine, Konkuk University Medical Center, Seoul, Korea
- KMID: 2512716
- DOI: http://doi.org/10.3343/alm.2021.41.4.372
Abstract
- Background
Accurate serum creatinine (Cr) concentration measurement is essential for evaluating kidney function. In 2011, the Korean Association of External Quality Assessment Service (KEQAS) launched an accuracy-based Cr proficiency testing (ABCr PT) survey. We analyzed long-term data of the KEQAS ABCr PT survey collected between 2011 and 2019 to assess recent trends in Cr assays in Korea.
Methods
The ABCr PT survey including three commutable fresh-frozen serum samples was performed twice a year. The target Cr concentration was assigned using isotope-dilution mass spectrometry. We analyzed data obtained from the participating laboratories, calculated the yearly bias, and evaluated bias trends for the major reagents and instruments. Outliers were excluded from all analysis.
Results
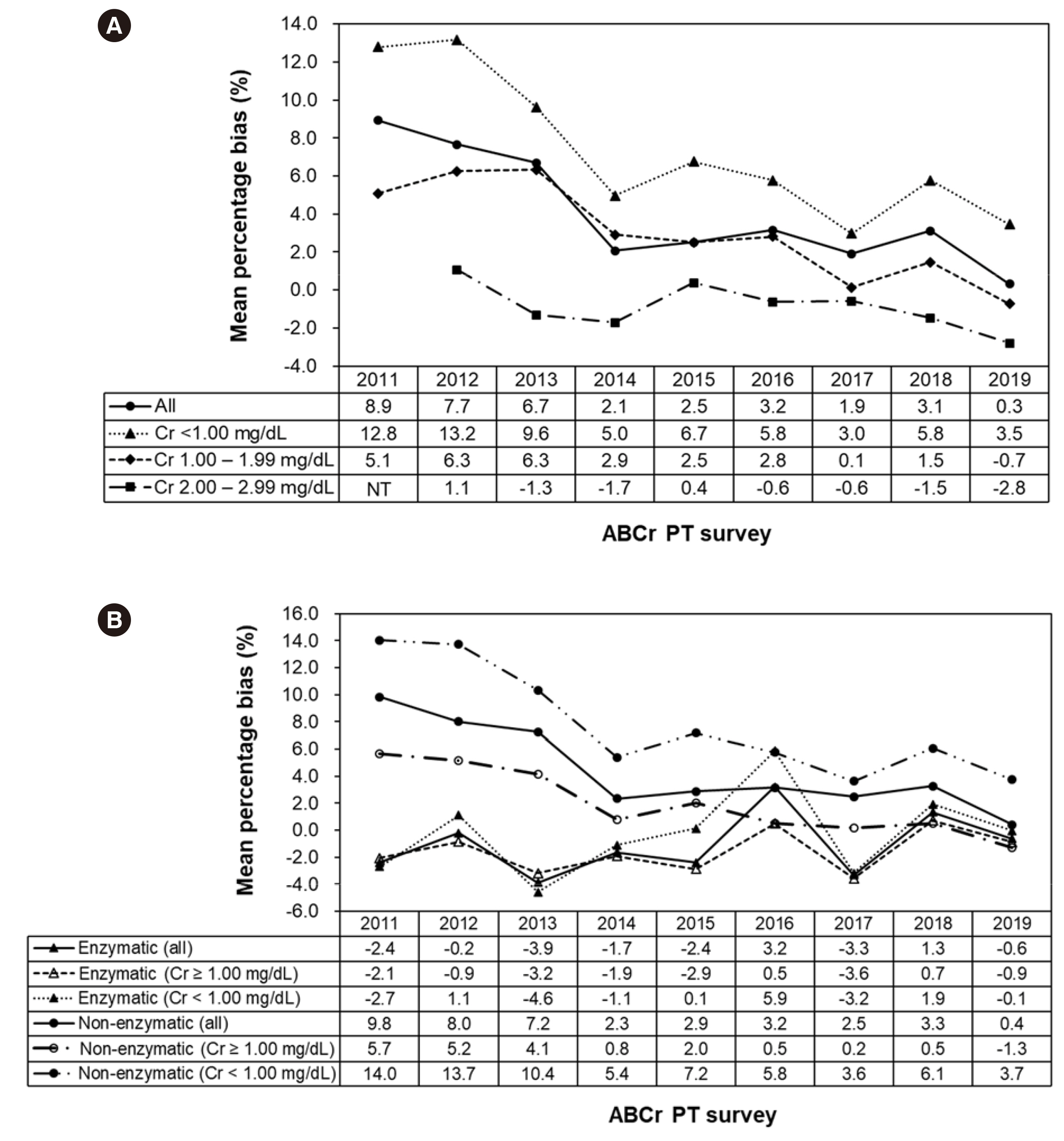
The mean percentage bias based on the total data of all participating laboratories was 10.8% in the 2011-A survey and 0.2% in 2019-B survey. Bias for the major reagents and instruments differed depending on the manufacturer. Enzymatic assays generally showed desirable bias ranging from –3.9% to 3.2% at all Cr concentrations and lower interlaboratory variability than non-enzymatic assays (enzymatic vs. non-enzymatic, 3.3%– 7.2% vs. 6.3%–9.1%).
Conclusions
Although the mean percentage bias of Cr assays tends to decrease over time, it is necessary to continuously strive to improve Cr assay accuracy, especially at low concentrations.
Keyword
Figure
Cited by 5 articles
-
Proposed Model for Evaluating Real-world Laboratory Results for Big Data Research
Sollip Kim, Eun-Jung Cho, Tae-Dong Jeong, Hyung-Doo Park, Yeo-Min Yun, Kyunghoon Lee, Yong-Wha Lee, Sail Chun, Won-Ki Min
Ann Lab Med. 2023;43(1):104-107. doi: 10.3343/alm.2023.43.1.104.Evaluation of automated calibration and quality control processes using the Aptio total laboratory automation system
Namhee Kim, Yein Kim, Jeongeun Park, Jungsoo Choi, Hyunyong Hwang
Kosin Med J. 2022;37(4):342-353. doi: 10.7180/kmj.22.144.Accuracy of the New Creatinine-based Equations for Estimating Glomerular Filtration Rate in Koreans
Tae-Dong Jeong, Jinyoung Hong, Woochang Lee, Sail Chun, Won-Ki Min
Ann Lab Med. 2023;43(3):244-252. doi: 10.3343/alm.2023.43.3.244.Laboratory Data Quality Evaluation in the Big Data Era
Sollip Kim
Ann Lab Med. 2023;43(5):399-400. doi: 10.3343/alm.2023.43.5.399.Current Status of Standardization of Glomerular Filtration Rate Markers in Korea
Tae-Dong Jeong
Ann Lab Med. 2025;45(3):272-275. doi: 10.3343/alm.2024.0702.
Reference
-
1. Stevens LA, Coresh J, Greene T, Levey AS. 2006; Assessing kidney function-measured and estimated glomerular filtration rate. N Engl J Med. 354:2473–83. DOI: 10.1056/NEJMra054415. PMID: 16760447.2. Kidney disease: Improving Global Outcomes (KDIGO) CKD Work Group. 2013; KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 3:1–150.3. Jeong TD, Cho EJ, Lee W, Chun S, Hong KS, Min WK. 2017; Accuracy assessment of five equations used for estimating the glomerular filtration rate in Korean adults. Ann Lab Med. 37:371–80. DOI: 10.3343/alm.2017.37.5.371. PMID: 28643485. PMCID: PMC5500735.
Article4. Park S, Jeong TD. 2019; Estimated glomerular filtration rates show minor but significant differences between the single and subgroup creatinine-based chronic kidney disease epidemiology collaboration equations. Ann Lab Med. 39:205–8. DOI: 10.3343/alm.2019.39.2.205. PMID: 30430784. PMCID: PMC6240521.
Article5. Kidney disease: Improving global outcomes (KDIGO) Acute Kidney Injury Work Group. 2012; KDIGO clinical practice guideline for acute kidney injury. Kidney Int Suppl. 2:1–138.6. Levey AS, Coresh J, Balk E, Kausz AT, Levin A, Steffes MW, et al. 2003; National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 139:137–47. DOI: 10.7326/0003-4819-139-2-200307150-00013. PMID: 12859163.
Article7. Miller WG. 2009; The role of proficiency testing in achieving standardization and harmonization between laboratories. Clin Biochem. 42:232–5. DOI: 10.1016/j.clinbiochem.2008.09.004. PMID: 19863911.
Article8. Miller WG, Jones GR, Horowitz GL, Weykamp C. 2011; Proficiency testing/external quality assessment: current challenges and future directions. Clin Chem. 57:1670–80. DOI: 10.1373/clinchem.2011.168641. PMID: 21965556.
Article9. Jeong TD, Lee HA, Lee K, Yun YM. 2019; Accuracy-based proficiency testing of creatinine measurement: 7 years' experience in Korea. J Lab Med Qual Assur. 41:13–23. DOI: 10.15263/jlmqa.2019.41.1.13.
Article10. Killeen AA, Ashwood ER, Ventura CB, Styer P. 2013; Recent trends in performance and current state of creatinine assays. Arch Pathol Lab Med. 137:496–502. DOI: 10.5858/arpa.2012-0134-CP. PMID: 23544939.
Article11. CLSI. 1999. Preparation and validation of commutable frozen human serum pools as secondary reference materials for cholesterol measurement procedures; approved guideline. CLSI C37-A. Clinical and Laboratory Standards Institute;Wayne, PA:12. Carobene A, Ceriotti F, Infusino I, Frusciante E, Panteghini M. 2014; Evaluation of the impact of standardization process on the quality of serum creatinine determination in Italian laboratories. Clin Chim Acta. 427:100–6. DOI: 10.1016/j.cca.2013.10.001. PMID: 24144863.
Article13. Helmersson-Karlqvist J, Ridefelt P, Boija EE, Nordin G. 2019; Lower creatinine concentration values and lower inter-laboratory variation among Swedish hospital laboratories in 2014 compared to 1996: results from the Equalis external quality assessment program. Clin Chem Lab Med. 57:838–44. DOI: 10.1515/cclm-2018-0670. PMID: 30982002.
Article14. Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. 2007; Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem. 53:766–72. DOI: 10.1373/clinchem.2006.077180. PMID: 17332152.
Article15. Stevens LA, Manzi J, Levey AS, Chen J, Deysher AE, Greene T, et al. 2007; Impact of creatinine calibration on performance of GFR estimating equations in a pooled individual patient database. Am J Kidney Dis. 50:21–35. DOI: 10.1053/j.ajkd.2007.04.004. PMID: 17591522.
Article16. Myers GL, Miller WG, Coresh J, Fleming J, Greenberg N, Greene T, et al. 2006; Recommendations for improving serum creatinine measurement: a report from the Laboratory Working Group of the National Kidney Disease Education Program. Clin Chem. 52:5–18. DOI: 10.1373/clinchem.2005.0525144. PMID: 16332993.
Article17. Aarsand A, Fernandez-Calle P, Webster C, Coskun A, Gonzales-Lao E, Diaz-Garzon J, et al. The EFLM Biological Variation Database. https://biologicalvariation.eu/. Updated on 27 Apr 2020.18. Cobbaert CM, Baadenhuijsen H, Weykamp CW. 2009; Prime time for enzymatic creatinine methods in pediatrics. Clin Chem. 55:549–58. DOI: 10.1373/clinchem.2008.116863. PMID: 19168555.
Article19. Piéroni L, Delanaye P, Boutten A, Bargnoux AS, Rozet E, Delatour V, et al. 2011; A multicentric evaluation of IDMS-traceable creatinine enzymatic assays. Clin Chim Acta. 412:2070–5. DOI: 10.1016/j.cca.2011.07.012. PMID: 21803031.
Article20. Drion I, Cobbaert C, Groenier KH, Weykamp C, Bilo HJ, Wetzels JF, et al. 2012; Clinical evaluation of analytical variations in serum creatinine measurements: why laboratories should abandon Jaffe techniques. BMC Nephrol. 13:133. DOI: 10.1186/1471-2369-13-133. PMID: 23043743. PMCID: PMC3504563.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Accuracy-Based Proficiency Testing of Creatinine Measurement: 7 Years' Experience in Korea
- Accuracy of Creatinine Assay According to Expanded Proficiency Testing in Participants
- Annual Report on the Results of Accuracy-Based Creatinine Proficiency Testing in Korea (2023) by the Korean Association of External Quality Assessment Service
- Impact of Type A Standard Measurement Uncertainty in the Primary Reference Measurement Procedure for Creatinine Assay on Bias Analysis in Accuracy-Based Creatinine Proficiency Testing
- Annual Report of the Korean Association of External Quality Assessment Service on Accuracy-Based Creatinine Testing (2022)