Ann Lab Med.
2021 Jul;41(4):366-371. 10.3343/alm.2021.41.4.366.
Standardization Status of Total Cholesterol Concentration Measurement: Analysis of Korean External Quality Assessment Data
- Affiliations
-
- 1Department of Laboratory Medicine, Soonchunhyang University Cheonan Hospital, Soonchunhyang University College of Medicine, Cheonan, Korea
- 2Department of Laboratory Medicine and Genetics, Soonchunhyang University Bucheon Hospital, Soonchunhyang University College of Medicine, Bucheon, Korea
- 3Department of Laboratory Medicine Inje University, Ilsan Paik Hospital, Goyang, Korea
- 4Department of Laboratory Medicine, Seoul National University Bundang Hospital and College of Medicine, Seongnam, Korea
- 5Department of Laboratory Medicine and Genetics, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Korea
- 6Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- KMID: 2512715
- DOI: http://doi.org/10.3343/alm.2021.41.4.366
Abstract
- Background
Total cholesterol concentration measurement is important in the diagnosis of dyslipidemia and evaluation of cardiovascular disease risk factors. Measurement reliability for obtaining an accurate total cholesterol concentration requires procedure standardization. We evaluated the standardization status for total cholesterol concentration measurement through Korean external quality assessment (EQA) data analysis.
Methods
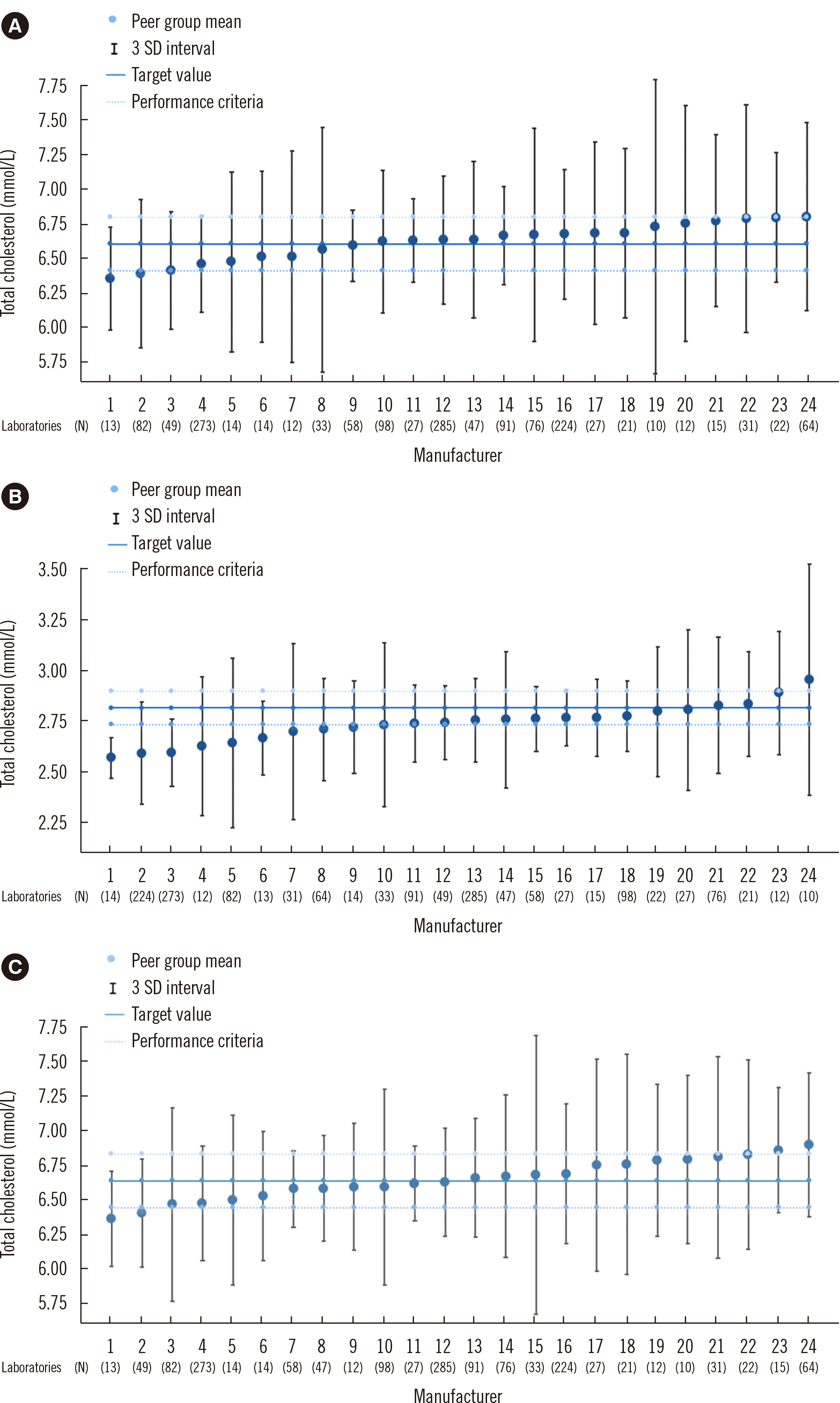
This study involved 1,670 laboratories that participated in the EQA of total cholesterol concentration measurements in 2019 for 32 products from different manufacturers. The target concentrations of three quality control (QC) materials (samples A, B, and C) were measured using the reference method and compared with EQA data. The performance criteria for total cholesterol concentration measurement were based on the National Cholesterol Education Program guidelines, with ± 3% inaccuracy.
Results
The target values and inaccuracies of the QC material based on the reference method measurements were 254.65 ± 7.64, 108.30 ± 3.25, and 256.29 ± 7.69 mg/dL (6.59 ± 0.20, 2.80 ± 0.08, and 6.63 ± 0.20 mmol/L) for samples A, B, and C, respectively. The performance criteria were not met in 42.7% laboratories for sample A, 68.4% of laboratories for sample B, and 38.0% laboratories for sample C.
Conclusions
Despite significant efforts to accurately measure total cholesterol concentrations, further actions are needed for measurement standardization. Manufacturers reporting values that differ from target values should check calibrator traceability; additional efforts to accurately measure total cholesterol concentrations are required for laboratories that use products from these manufacturers.
Figure
Cited by 3 articles
-
Evaluation of automated calibration and quality control processes using the Aptio total laboratory automation system
Namhee Kim, Yein Kim, Jeongeun Park, Jungsoo Choi, Hyunyong Hwang
Kosin Med J. 2022;37(4):342-353. doi: 10.7180/kmj.22.144.Laboratory Data Quality Evaluation in the Big Data Era
Sollip Kim
Ann Lab Med. 2023;43(5):399-400. doi: 10.3343/alm.2023.43.5.399.Quality Status of the Preanalytical Phase of Clinical Laboratories in Korea
Yuna Choi, Kyunghoon Lee, Hyun-Jung Choi, Soo Young Moon, Jinsook Lim, Sollip Kim
Lab Med Online. 2024;14(2):90-99. doi: 10.47429/lmo.2024.14.2.90.
Reference
-
1. National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). 2002; Third report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 106:3143–421. DOI: 10.1161/circ.106.25.3143. PMID: 12485966.2. Cholesterol Reference Method Laboratory Network. Total cholesterol certification protocol for manufacturers. http://www.cdc.gov/labstandards/pdf/crmln/RevisedTCprotocolOct04.pdf. Updated on Apr 8, 2020.3. Miller WG. 2009; The role of proficiency testing in achieving standardization and harmonization between laboratories. Clin Biochem. 42:232–5. DOI: 10.1016/j.clinbiochem.2008.09.004. PMID: 19863911.
Article4. Kim S, Lee K, Park HD, Lee YW, Chun S, Min WK. 2021; Schemes and performance evaluation criteria of Korean Association of External Quality Assessment (KEQAS) for improving laboratory testing. Ann Lab Med. 41:230–9. DOI: 10.3343/alm.2021.41.2.230. PMID: 33063686.
Article5. Kim H, Kim S, Yun YM, Um TH, Chang J, Lee KS, et al. 2020; Status of quality control for laboratory tests of medical institutions in Korea: analysis of 10 years of data on external quality assessment participation. Healthcare (Basel). 8:75. DOI: 10.3390/healthcare8020075. PMID: 32230819. PMCID: PMC7349217.
Article6. Sundvall J, Leiviskä J, Alfthan G, Vartiainen E. 2007; Serum cholesterol during 27 years: assessment of systematic error and affecting factors and their role in interpreting population trends. Clin Chim Acta. 378:93–8. DOI: 10.1016/j.cca.2006.10.021. PMID: 17169352.7. Ross JW, Miller WG, Myers GL, Praestgaard J. 1998; The accuracy of laboratory measurements in clinical chemistry: a study of 11 routine chemistry analytes in the College of American Pathologist Chemistry Survey with fresh frozen serum, definitive methods, and reference methods. Arch Pathol Lab Med. 122:587–608.8. Ellerbe P, Myers GL, Cooper GR, Hertz HS, Sniegoski LT, Welch MJ, et al. 1990; A comparison of results for cholesterol in human serum obtained by the Reference Method and by the Definitive Method of the National Reference System for cholesterol. Clin Chem. 36:370–5. DOI: 10.1093/clinchem/36.2.370. PMID: 2302783.
Article9. Abel LL, Levy BB, Brodie BB, Kendall FE. 1952; A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 195:357–66. DOI: 10.1016/S0021-9258(19)50907-3.10. Bowers GN, Jr , Fassett JD, White E 5th. 1993; Isotope dilution mass spectrometry and the National Reference System. Anal Chem. 65:475R–9R. DOI: 10.1021/ac00060a620. PMID: 8333621.
Article11. Edwards SH, Kimberly MM, Pyatt SD, Stribling SL, Dobbin KD, Myers GL. 2011; Proposed serum cholesterol reference measurement procedure by gas chromatography-isotope dilution mass spectrometry. Clin Chem. 57:614–22. DOI: 10.1373/clinchem.2010.158766. PMID: 21317273.
Article12. Warnick GR, Kimberly MM, Waymack PP, Leary ET, Myers GL. 2008; Standardization of measurements for cholesterol, triglycerides, and major lipoproteins. Lab Med. 39:481–90. DOI: 10.1309/6UL9RHJH1JFFU4PY.
Article13. Long Q, Qi T, Zhang T, Wang J, Zeng J, Yan Y, et al. 2021; Commutability assessment of candidate external quality assessment materials for aminotransferase approaches in China. Ann Lab Med. 41:68–76. DOI: 10.3343/alm.2021.41.1.68. PMID: 32829581. PMCID: PMC7443529.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- HbA1c: A Review of Analytical and Clinical Aspects
- Quantitative Evaluation of the Real-World Harmonization Status of Laboratory Test Items Using External Quality Assessment Data
- A Study of the Standardization and the External Quality Assessment for Antinuclear Antibody, Anti-Double-Stranded DNA, and AntiExtractable Nuclear Antigen Antibody Testing
- A New Strategy for Evaluating the Quality of Laboratory Results for Big Data Research: Using External Quality Assessment Survey Data (2010–2020)
- Standardization of ABO Antibody Titer Measurement at Laboratories in Korea