Ann Lab Med.
2021 May;41(3):302-309. 10.3343/alm.2021.41.3.302.
Diagnostic Efficacy of Serum Mac-2 Binding Protein Glycosylation Isomer and Other Markers for Liver Fibrosis in Non-Alcoholic Fatty Liver Diseases
- Affiliations
-
- 1Department of Internal Medicine, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- 2Department of Biochemistry and Cell Biology, Cell and Matrix Research Institute, School of Medicine, Kyungpook National University, Daegu, Korea
- 3Department of Pathology, School of Medicine, Kyungpook National University, Kyungpook National University Hospital, Daegu, Korea
- 4Department of Medical Informatics, School of Medicine, Kyungpook National University, Daegu, Korea
- KMID: 2512688
- DOI: http://doi.org/10.3343/alm.2021.41.3.302
Abstract
- Background
Mac-2 binding protein glycosylation isomer (M2BPGi) has been established as a non-invasive biomarker for liver fibrosis. We evaluated the diagnostic efficacy of M2BPGi compared with those of other liver fibrosis markers in liver fibrosis in non-alcoholic fatty liver disease (NAFLD).
Methods
We analyzed serum M2BPGi levels in 113 NAFLD patients. A pathologist graded liver fibrosis histopathologically. The diagnostic efficacies of serum M2BPGi and other liver fibrosis markers (aspartate aminotransferase to platelet ratio index, fibrosis index based on four factors, and NAFLD fibrosis score [NFS]) were evaluated using correlation, area under the ROC curve (AUC), logistic regression, and C-statistics.
Results
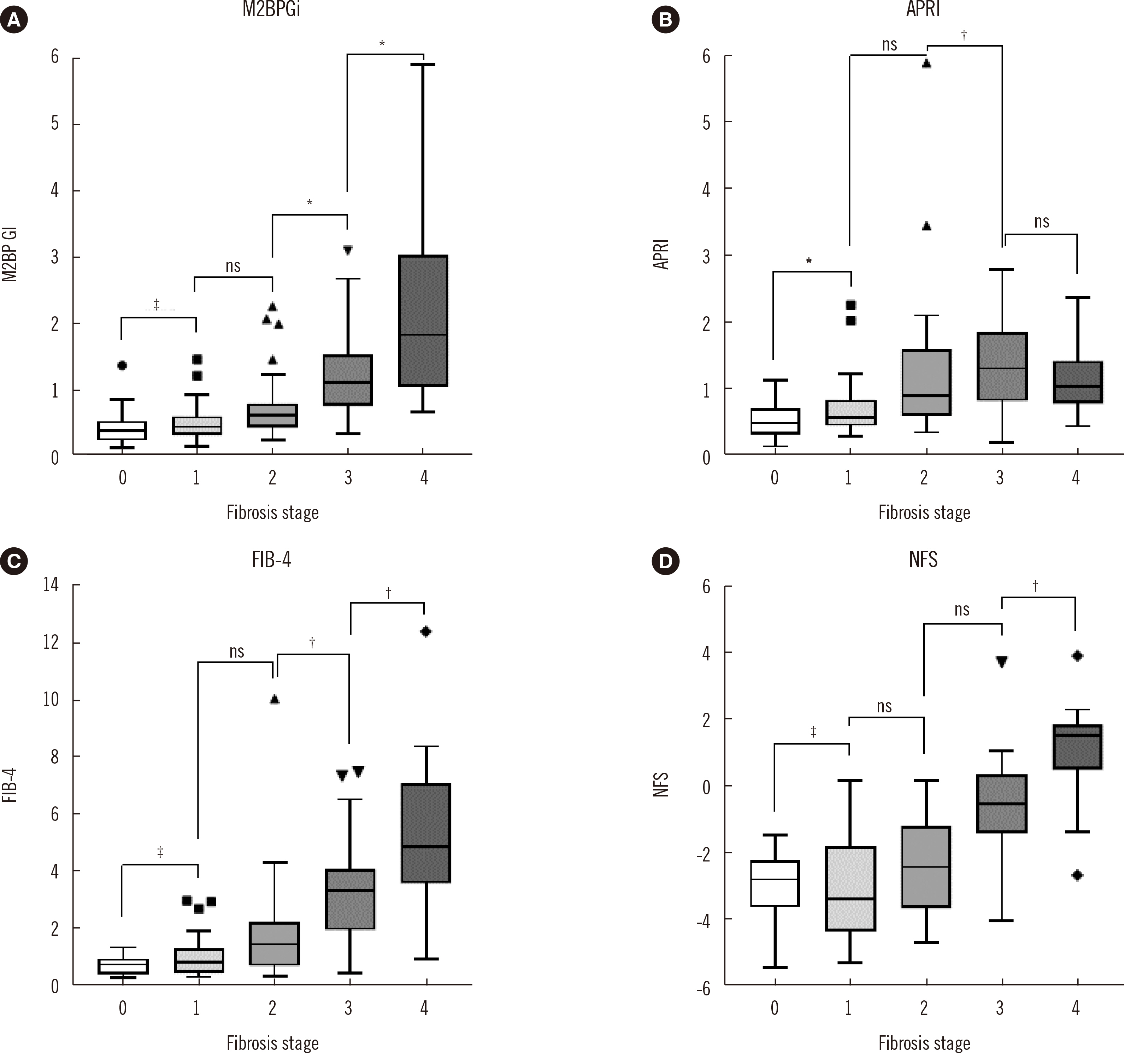
Serum M2BPGi level and other liver fibrosis markers showed a moderate correlation with fibrosis grade. The AUC values of M2BPGi were 0.761, 0.819, 0.866, and 0.900 for diagnosing fibrosis (F) > 0, F > 1, F > 2, and F > 3, respectively. Logistic regression analysis showed M2BPGi as the only independent factor associated with F > 2 and F > 3. Although C-statistics showed that NFS was the best diagnostic factor for F > 2 and F > 3, M2BPGi with NFS had an increased C-statistics value, indicating that it is a better diagnostic model.
Conclusions
The serum M2BPGi level increased with liver fibrosis severity and could be a good biomarker for diagnosing advanced fibrosis and cirrhosis in NAFLD patients. A well-controlled, prospective study with a larger sample size is needed to validate the diagnostic power of M2BPGi and other fibrosis markers in NAFLD.
Keyword
Figure
Cited by 1 articles
-
Comparison of Non-Invasive Clinical Algorithms for Liver Fibrosis in Patients With Chronic Hepatitis B to Reduce the Need for Liver Biopsy: Application of Enhanced Liver Fibrosis and Mac-2 Binding Protein Glycosylation Isomer
Mina Hur, Mikyoung Park, Hee-Won Moon, Won Hyeok Choe, Chae Hoon Lee
Ann Lab Med. 2022;42(2):249-257. doi: 10.3343/alm.2022.42.2.249.
Reference
-
1. Mak LY, Ko M, To E, Wong DK, Ma JH, Hui TL, et al. 2019; Serum Mac-2-binding protein glycosylation isomer and risk of hepatocellular carcinoma in entecavir-treated chronic hepatitis B patients. J Gastroenterol Hepatol. 34:1817–23. DOI: 10.1111/jgh.14637. PMID: 30786068.
Article2. Shinkai N, Nojima M, Iio E, Matsunami K, Toyoda H, Murakami S, et al. 2018; High levels of serum Mac-2-binding protein glycosylation isomer (M2BPGi) predict the development of hepatocellular carcinoma in hepatitis B patients treated with nucleot(s)ide analogues. J Gastroenterol. 53:883–9. DOI: 10.1007/s00535-017-1424-0. PMID: 29288305.
Article3. Nagata H, Nakagawa M, Nishimura-Sakurai Y, Asano Y, Tsunoda T, Miyoshi M, et al. 2016; Serial measurement of Wisteria floribunda agglutinin positive Mac-2-binding protein is useful for predicting liver fibrosis and the development of hepatocellular carcinoma in chronic hepatitis C patients treated with IFN-based and IFN-free therapy. Hepatol Int. 10:956–64. DOI: 10.1007/s12072-016-9754-1. PMID: 27435935.4. Kawanaka M, Tomiyama Y, Hyogo H, Koda M, Shima T, Tobita H, et al. 2018; Wisteria floribunda agglutinin-positive Mac-2 binding protein predicts the development of hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. Hepatol Res. 48:521–8. DOI: 10.1111/hepr.13054. PMID: 29316028.5. Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. 2016; Clinical significance of serum Wisteria floribunda agglutinin positive Mac-2-binding protein level and high-sensitivity C-reactive protein concentration in autoimmune hepatitis. Hepatol Res. 46:613–21. DOI: 10.1111/hepr.12596. PMID: 26406984.6. Umemura T, Joshita S, Sekiguchi T, Usami Y, Shibata S, Kimura T, et al. 2015; Serum Wisteria floribunda agglutinin-positive Mac-2-Binding protein level predicts liver fibrosis and prognosis in primary biliary cirrhosis. Am J Gastroenterol. 110:857–64. DOI: 10.1038/ajg.2015.118. PMID: 25916223.7. Nishikawa H, Enomoto H, Iwata Y, Hasegawa K, Nakano C, Takata R, et al. 2016; Impact of serum Wisteria floribunda agglutinin positive Mac-2-binding protein and serum interferon-gamma-inducible protein-10 in primary biliary cirrhosis. Hepatol Res. 46:575–83. DOI: 10.1111/hepr.12595. PMID: 26418076.8. Tamaki N, Higuchi M, Kurosaki M, Kirino S, Osawa L, Watakabe K, et al. 2019; Wisteria floribunda agglutinin-positive mac-2 binding protein as an age-independent fibrosis marker in nonalcoholic fatty liver disease. Sci Rep. 9:10109. DOI: 10.1038/s41598-019-46172-1. PMID: 31300805. PMCID: PMC6626055.
Article9. Abe M, Miyake T, Kuno A, Imai Y, Sawai Y, Hino K, et al. 2015; Association between Wisteria floribunda agglutinin-positive Mac-2 binding protein and the fibrosis stage of non-alcoholic fatty liver disease. J Gastroenterol. 50:776–84. DOI: 10.1007/s00535-014-1007-2. PMID: 25326152.10. Jekarl DW, Choi H, Lee S, Kwon JH, Lee SW, Yu H, et al. 2018; Diagnosis of liver fibrosis with Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA-M2BP) among chronic hepatitis B patients. Ann Lab Med. 38:348–54. DOI: 10.3343/alm.2018.38.4.348. PMID: 29611385. PMCID: PMC5895864.11. Kim M, Jun DW, Park H, Kang BK, Sumida Y. 2020; Sequential combination of FIB-4 followed by M2BPGi enhanced diagnostic performance for advanced hepatic fibrosis in an average risk population. J Clin Med. 9:1119. DOI: 10.3390/jcm9041119. PMID: 32295166. PMCID: PMC7230806.
Article12. Nah EH, Cho S, Kim S, Kim HS, Cho HI. 2020; Diagnostic performance of Mac-2 binding protein glycosylation isomer (M2BPGi) in screening liver fibrosis in health checkups. J Clin Lab Anal. 34:e23316. DOI: 10.1002/jcla.23316. PMID: 32227396. PMCID: PMC7439422.
Article13. Moon HW, Park M, Hur M, Kim H, Choe WH, Yun YM. 2018; Usefulness of enhanced liver fibrosis, glycosylation isomer of Mac-2 binding protein, galectin-3, and soluble suppression of tumorigenicity 2 for assessing liver fibrosis in chronic liver diseases. Ann Lab Med. 38:331–7. DOI: 10.3343/alm.2018.38.4.331. PMID: 29611383. PMCID: PMC5895862.
Article14. Younossi ZM, Blissett D, Blissett R, Henry L, Stepanova M, Younossi Y, et al. 2016; The economic and clinical burden of nonalcoholic fatty liver disease in the United States and Europe. Hepatology. 64:1577–86. DOI: 10.1002/hep.28785. PMID: 27543837.
Article15. Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. 2016; Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 64:73–84. DOI: 10.1002/hep.28431. PMID: 26707365.
Article16. Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. 2019; A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 16:589–604. DOI: 10.1038/s41575-019-0186-y. PMID: 31439937. PMCID: PMC6813818.
Article17. Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, et al. 2002; Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 97:2614–8. DOI: 10.1111/j.1572-0241.2002.06038.x. PMID: 12385448.
Article18. Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. 2005; Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 41:1313–21. DOI: 10.1002/hep.20701. PMID: 15915461.
Article19. Toshima T, Shirabe K, Ikegami T, Yoshizumi T, Kuno A, Togayachi A, et al. 2015; A novel serum marker, glycosylated Wisteria floribunda agglutinin-positive Mac-2 binding protein (WFA(+)-M2BP), for assessing liver fibrosis. J Gastroenterol. 50:76–84. DOI: 10.1007/s00535-014-0946-y. PMID: 24603981.20. Yamasaki K, Tateyama M, Abiru S, Komori A, Nagaoka S, Saeki A, et al. 2014; Elevated serum levels of Wisteria floribunda agglutinin-positive human Mac-2 binding protein predict the development of hepatocellular carcinoma in hepatitis C patients. Hepatology. 60:1563–70. DOI: 10.1002/hep.27305. PMID: 25042054. PMCID: PMC4278450.21. Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, et al. 2003; A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 38:518–26. DOI: 10.1053/jhep.2003.50346. PMID: 12883497.
Article22. Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. 2006; Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 43:1317–25. DOI: 10.1002/hep.21178. PMID: 16729309.
Article23. Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. 2007; The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 45:846–54. DOI: 10.1002/hep.21496. PMID: 17393509.
Article24. DeLong ER, DeLong DM, Clarke-Pearson DL. 1988; Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 44:837–45. DOI: 10.2307/2531595. PMID: 3203132.
Article25. Douglas CE, Michael FA. 1991; On distribution-free multiple comparisons in the one-way analysis of variance. Communications in Statistics - Theory and Methods. 20:127–39. DOI: 10.1080/03610929108830487.
Article26. Tamaki N, Kurosaki M, Loomba R, Izumi N. 2021; Clinical utility of Mac-2 Binding Protein Glycosylation Isomer in chronic liver diseases. Ann Lab Med. 41:16–24. DOI: 10.3343/alm.2021.41.1.16. PMID: 32829576. PMCID: PMC7443525.
Article27. Atsukawa M, Tsubota A, Okubo T, Arai T, Nakagawa A, Itokawa N, et al. 2018; Serum Wisteria floribunda agglutinin-positive Mac-2 binding protein more reliably distinguishes liver fibrosis stages in non-alcoholic fatty liver disease than serum Mac-2 binding protein. Hepatol Res. 48:424–32. DOI: 10.1111/hepr.13046. PMID: 29274190.28. European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. 2015; EASL-ALEH Clinical Practice Guidelines: non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 63:237–64. DOI: 10.1016/j.jhep.2015.04.006. PMID: 25911335.29. Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. 2017; Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 66:1486–501. DOI: 10.1002/hep.29302. PMID: 28586172.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Clinical Utility of Mac-2 Binding Protein Glycosylation Isomer in Chronic Liver Diseases
- Proposal of a Novel Serological Algorithm Combining FIB-4 and Serum M2BPGi for Advanced Fibrosis in Nonalcoholic Fatty Liver Disease
- Mac-2 Binding Protein Glycosylation Isomer: Emerging Non-Invasive Serum Marker for Liver Fibrosis
- Usefulness of Enhanced Liver Fibrosis, Glycosylation Isomer of Mac-2 Binding Protein, Galectin-3, and Soluble Suppression of Tumorigenicity 2 for Assessing Liver Fibrosis in Chronic Liver Diseases
- Mac-2 binding protein glycan isomer is new serum biomarker for assessing liver fibrosis: non-invasive method