Ann Lab Med.
2021 Mar;41(2):190-197. 10.3343/alm.2021.41.2.190.
Prediction of HLA-DQ in Deceased Donors and its Clinical Significance in Kidney Transplantation
- Affiliations
-
- 1Department of Laboratory Medicine, College of Medicine, Ewha Womans University, Seoul, Korea
- 2Department of Laboratory Medicine, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 3Division of Kidney and Pancreas Transplantation, Department of Surgery, University of Ulsan College of Medicine and Asan Medical Center, Seoul, Korea
- 4Department of Laboratory Medicine, College of Medicine, Korea University, Seoul, Korea
- KMID: 2512665
- DOI: http://doi.org/10.3343/alm.2021.41.2.190
Abstract
- Background
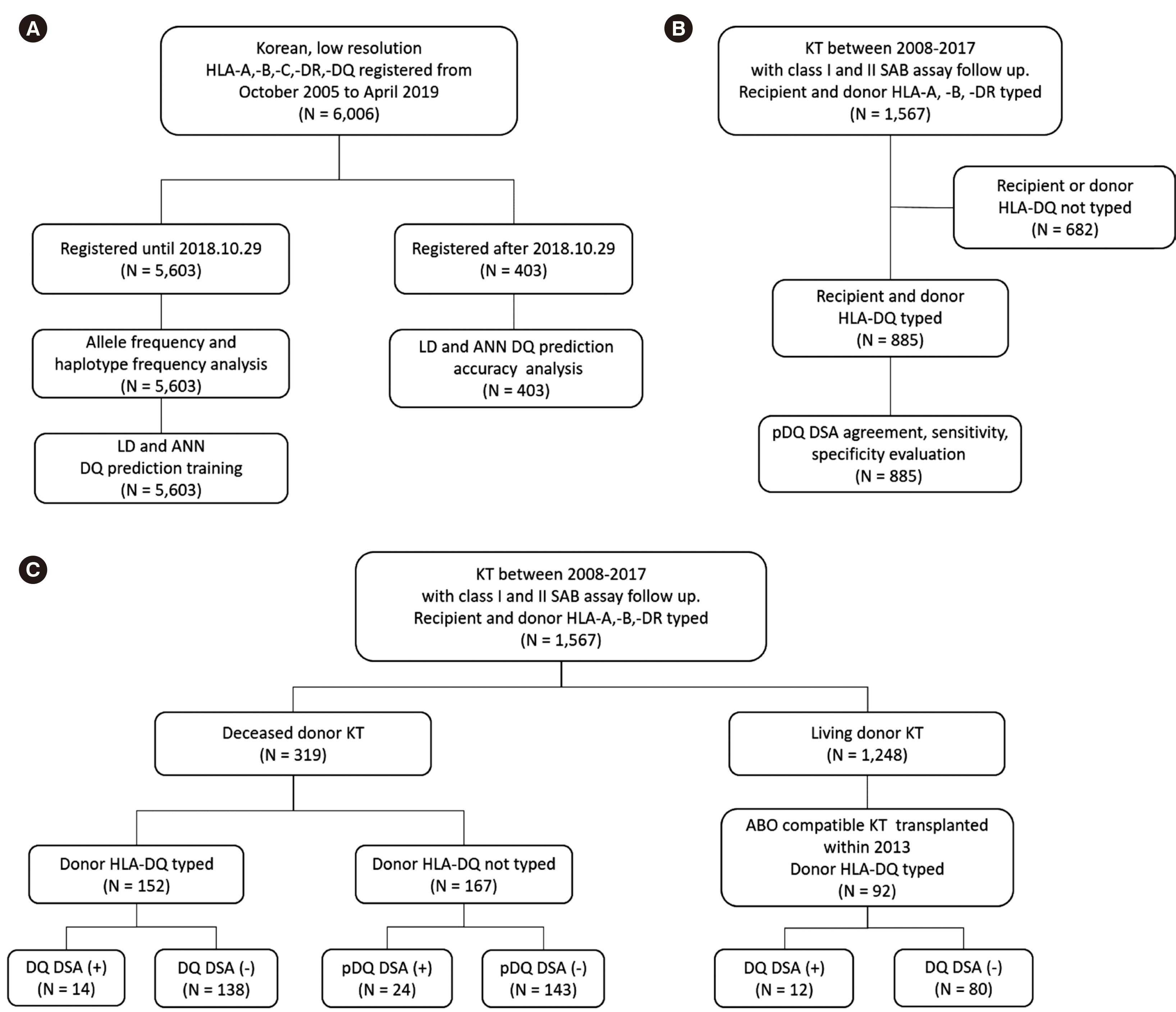
HLA-DQ typing in deceased donors is not mandatory in Korea. Therefore, when patients develop DQ antibodies after kidney transplantation (KT) from deceased donor, it is impossible to determine whether they are donor-specific antibodies (DSA). We developed DQ prediction programs for the HLA gene and evaluated their clinical utility.
Methods
Two HLA-DQ prediction programs were developed: one based on Lewontin’s linkage disequilibrium (LD) and haplotype frequency and the other on an artificial neural network (ANN). Low-resolution HLA-A, -B, -DR, and -DQ typing data of 5,603 Korean patients were analyzed in terms of haplotype frequency and used to develop an ANN DQ prediction program. Predicted DQ (pDQ) genotype accuracy was analyzed using the typed DQ data of 403 patients. pDQ DSA agreement, sensitivity, specificity, and false-negative rate was evaluated using 1,970 single-antigen bead assays performed on 885 KT recipients. The clinical significance of DQ and pDQ DSA was evaluated in 411 KT recipients.
Results
pDQ genotype accuracies were 75.4% (LD algorithm) and 75.7% (ANN). When the second most likely pDQ (LD algorithm) was also considered, the genotype accuracy increased to 92.6%. pDQ DSA (LD algorithm) agreement, sensitivity, specificity, and falsenegative rate were 97.5%, 97.3%, 98.6%, and 2.4%, respectively. The antibody-mediated rejection treatment frequency was significantly higher in DQ or pDQ DSA-positive patients than in DQ or pDQ DSA-negative patients (P < 0.001).
Conclusions
Our DQ prediction programs showed good accuracy and could aid DQ DSA detection in patients who had undergone deceased donor KT without donor HLA-DQ typing.
Keyword
Figure
Reference
-
1. Ozawa M, Rebellato LM, Terasaki PI, Tong A, Briley KP, Catrou P, et al. 2006; Longitudinal testing of 266 renal allograft patients for HLA and MICA antibodies: Greenville experience. Clin Transpl. 265–90. PMID: 18365384.2. DeVos JM, Gaber AO, Knight RJ, Land GA, Suki WN, Gaber LW, et al. 2012; Donor-specific HLA-DQ antibodies may contribute to poor graft outcome after renal transplantation. Kidney Int. 82:598–604. DOI: 10.1038/ki.2012.190. PMID: 22622504.
Article3. Willicombe M, Brookes P, Sergeant R, Santos-Nunez E, Steggar C, Galliford J, et al. 2012; De novo DQ donor-specific antibodies are associated with a significant risk of antibody-mediated rejection and transplant glomerulopathy. Transplantation. 94:172–7. DOI: 10.1097/TP.0b013e3182543950. PMID: 22735711.
Article4. Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. 2013; Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 95:410–7. DOI: 10.1097/TP.0b013e31827d62e3. PMID: 23380861.
Article5. Lee H, Min JW, Kim JI, Moon IS, Park KH, Yang CW, et al. 2016; Clinical significance of HLA-DQ antibodies in the development of chronic antibody-mediated rejection and allograft failure in kidney transplant recipients. Medicine (Baltimore). 95:e3094. DOI: 10.1097/MD.0000000000003094. PMID: 26986147. PMCID: PMC4839928.
Article6. Kobayashi T, Maruya E, Niwa M, Saji H, Kohara S, Katayama A, et al. 2011; Significant association between chronic antibody-mediated rejection and donor-specific antibodies against HLA-DRB rather than DQB in renal transplantation. Hum Immunol. 72:11–7. DOI: 10.1016/j.humimm.2010.10.018. PMID: 20974206.
Article7. Yu S, Huh HJ, Lee KW, Park JB, Kim SJ, Huh W, et al. 2020; Pre-transplant angiotensin II type 1 receptor antibodies and anti-endothelial cell antibodies predict graft function and allograft rejection in a low-risk kidney transplantation setting. Ann Lab Med. 40:398–408. DOI: 10.3343/alm.2020.40.5.398. PMID: 32311853. PMCID: PMC7169631.
Article8. Organ Procurement and Transplantation Network, OPTN Policies. https://optn.transplant.hrsa.gov/media/1200/optn_policies.pdf. Updated on Sep 2020.9. Eurotransplant, Eurotransplant Manual© version 4.6 Chapter 10 Histocompatibility testing in: Eurotransplant. Eurotransplant. http://www.eurotransplant.org/wp-content/uploads/2020/01/H10-Histocompatibility-v4.6.pdf. Updated on Feb 2020.10. Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, et al. 2002; The structure of haplotype blocks in the human genome. Science. 296:2225–9. DOI: 10.1126/science.1069424. PMID: 12029063.
Article11. Otegbeye F. 2017; Understanding population-wide haplotype frequencies of human leukocyte antigen (HLA) alleles in linkage disequilibrium is important for hematopoietic stem cell transplantation. Rev Bras Hematol Hemoter. 39:187–8. DOI: 10.1016/j.bjhh.2017.02.003. PMID: 28830592. PMCID: PMC5568582.
Article12. Slatkin M. 2008; Linkage disequilibrium--understanding the evolutionary past and mapping the medical future. Nat Rev Genet. 9:477–85. DOI: 10.1038/nrg2361. PMID: 18427557. PMCID: PMC5124487.13. Haas M, Loupy A, Lefaucheur C, Roufosse C, Glotz D, Seron D, et al. 2018; The Banff 2017 Kidney Meeting Report: Revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant. 18:293–307. DOI: 10.1111/ajt.14625. PMID: 29243394.
Article14. Navarrete C, Jaraquemada D, Fainboim L, Karr R, Hui K, Awad J, et al. 1985; Genetic and functional relationship of the HLA-DR and HLA-DQ antigens. Immunogenetics. 21:97–101. DOI: 10.1007/BF00372246. PMID: 3881342.
Article15. Bushell A, Higgins RM, Wood KJ, Morris PJ. 1989; HLA-DQ mismatches between donor and recipient in the presence of HLA-DR compatibility do not influence the function or outcome of renal transplants. Hum Immunol. 26:179–89. DOI: 10.1016/0198-8859(89)90037-2. PMID: 2575089.
Article16. Lim WH, Chapman JR, Coates PT, Lewis JR, Russ GR, Watson N, et al. 2016; HLA-DQ mismatches and rejection in kidney transplant recipients. Clin J Am Soc Nephrol. 11:875–83. DOI: 10.2215/CJN.11641115. PMID: 27034399. PMCID: PMC4858494.
Article17. Leeaphorn N, Pena JRA, Thamcharoen N, Khankin EV, Pavlakis M, Cardarelli F. 2018; HLA-DQ mismatching and kidney transplant outcomes. Clin J Am Soc Nephrol. 13:763–71. DOI: 10.2215/CJN.10860917. PMID: 29685925. PMCID: PMC5968890.
Article18. Freitas MCS, Rebellato LM, Ozawa M, Nguyen A, Sasaki N, Everly M, et al. 2013; The role of immunoglobulin-G subclasses and C1q in de novo HLA-DQ donor-specific antibody kidney transplantation outcomes. Transplantation. 95:1113–9. DOI: 10.1097/TP.0b013e3182888db6. PMID: 23514959.
Article19. Haas M, Sis B, Racusen LC, Solez K, Glotz D, Colvin RB, et al. 2014; Banff 2013 meeting report: inclusion of c4d-negative antibody-mediated rejection and antibody-associated arterial lesions. Am J Transplant. 14:272–83. DOI: 10.1111/ajt.12590. PMID: 24472190.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- Desensitization in HLA Incompatible Transplantation
- Clinical relevance and characteristics of pretransplant donor-specific anti-human leukocyte antigen-DQ antibodies in kidney transplantation
- The clinical impact of preformed human leukocyte antigen-DQ donor-specific antibodies on graft outcomes in kidney transplantation
- Effect of preexisting human leukocyte antigen donor-specific antibodies especially human leukocyte antigen-DQ on kidney transplant outcome
- Erratum: Pediatric kidney transplantation is different from adult kidney transplantation