J Bacteriol Virol.
2020 Dec;50(4):273-281. 10.4167/jbv.2020.50.4.273.
Semi-Replication-Competent Retroviral Vectors Expressing Gibbon Ape Leukemia Virus Fusogenic Membrane Glycoprotein (GALV FMG) Gene for Cancer Gene Therapy
- Affiliations
-
- 1Department of Microbiology, Dankook University, Cheonan 31116, Republic of Korea
- KMID: 2512149
- DOI: http://doi.org/10.4167/jbv.2020.50.4.273
Abstract
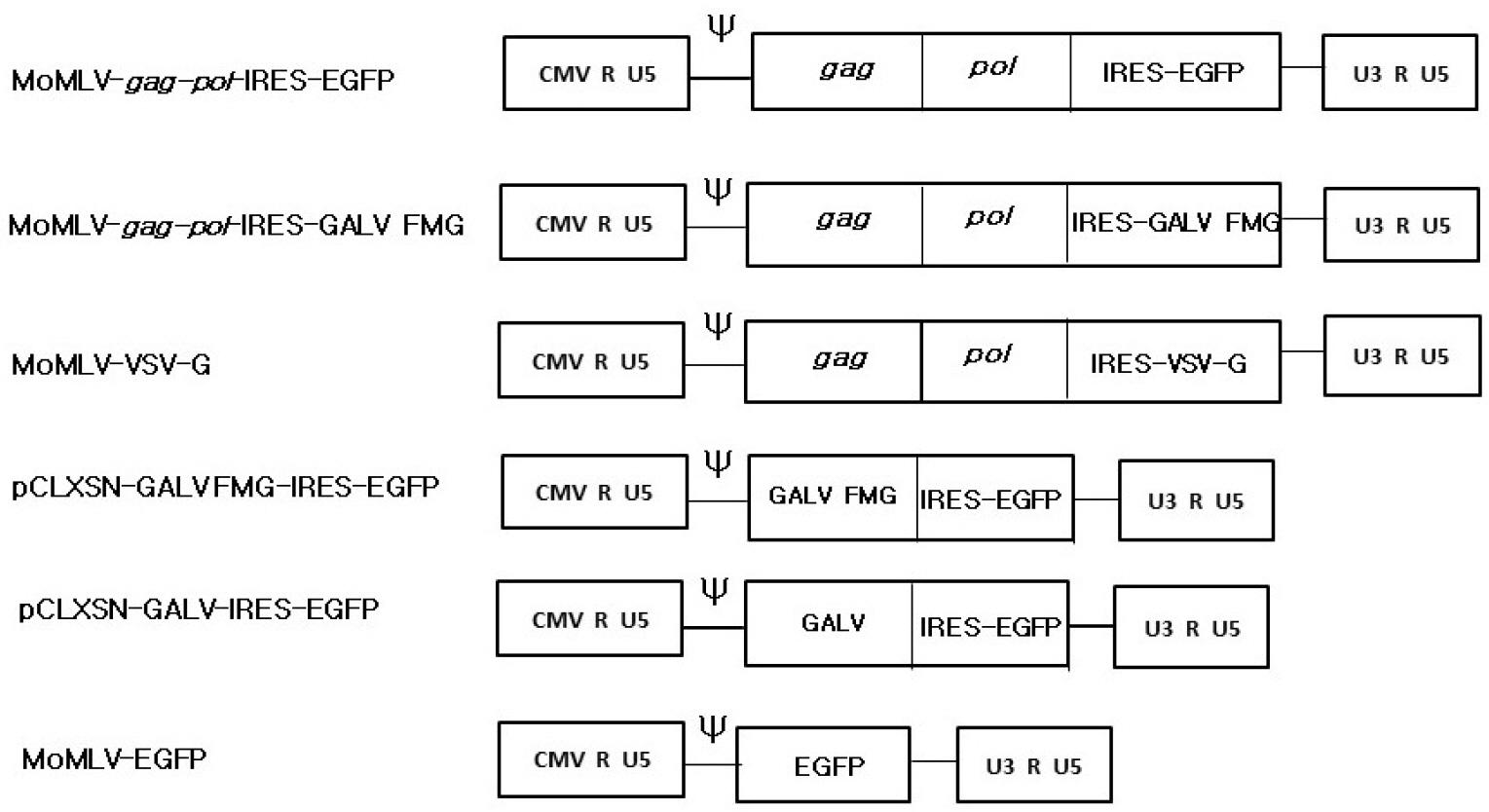
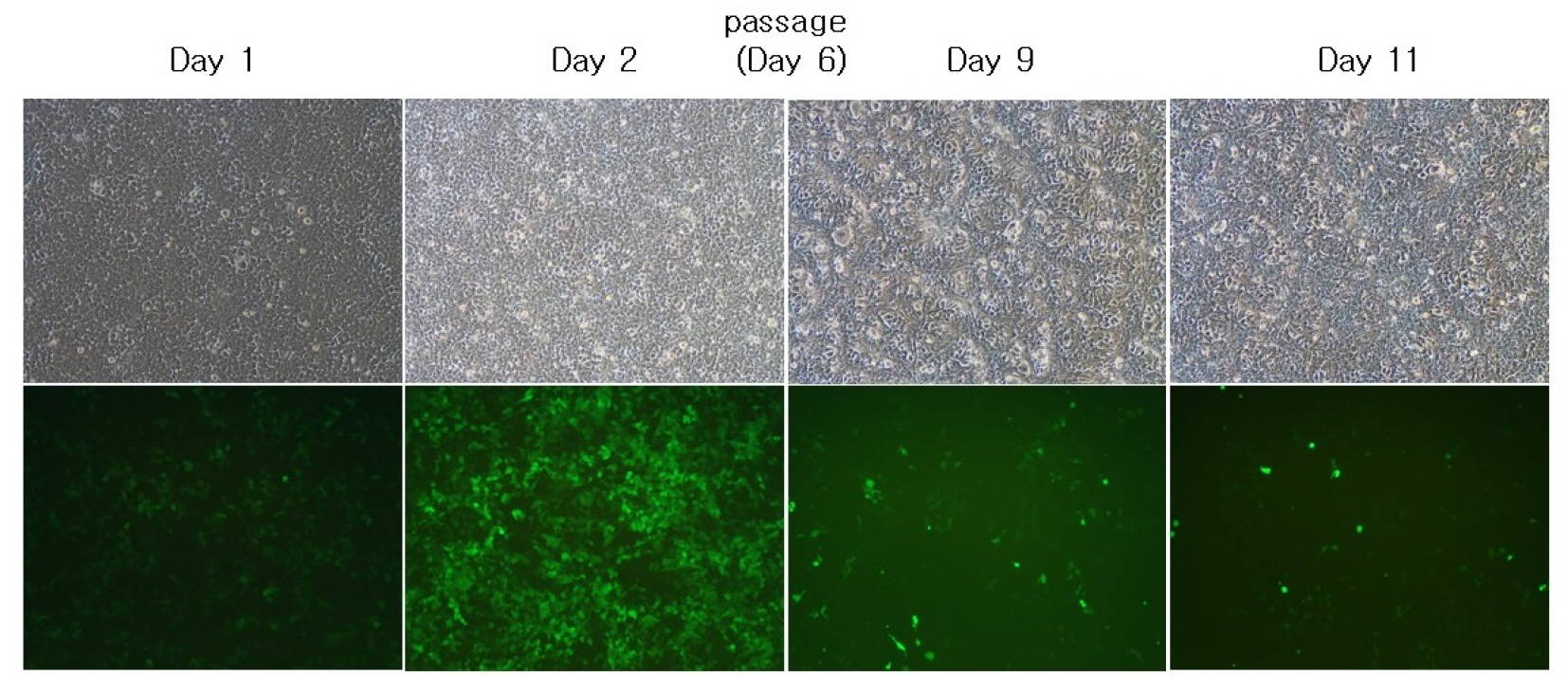
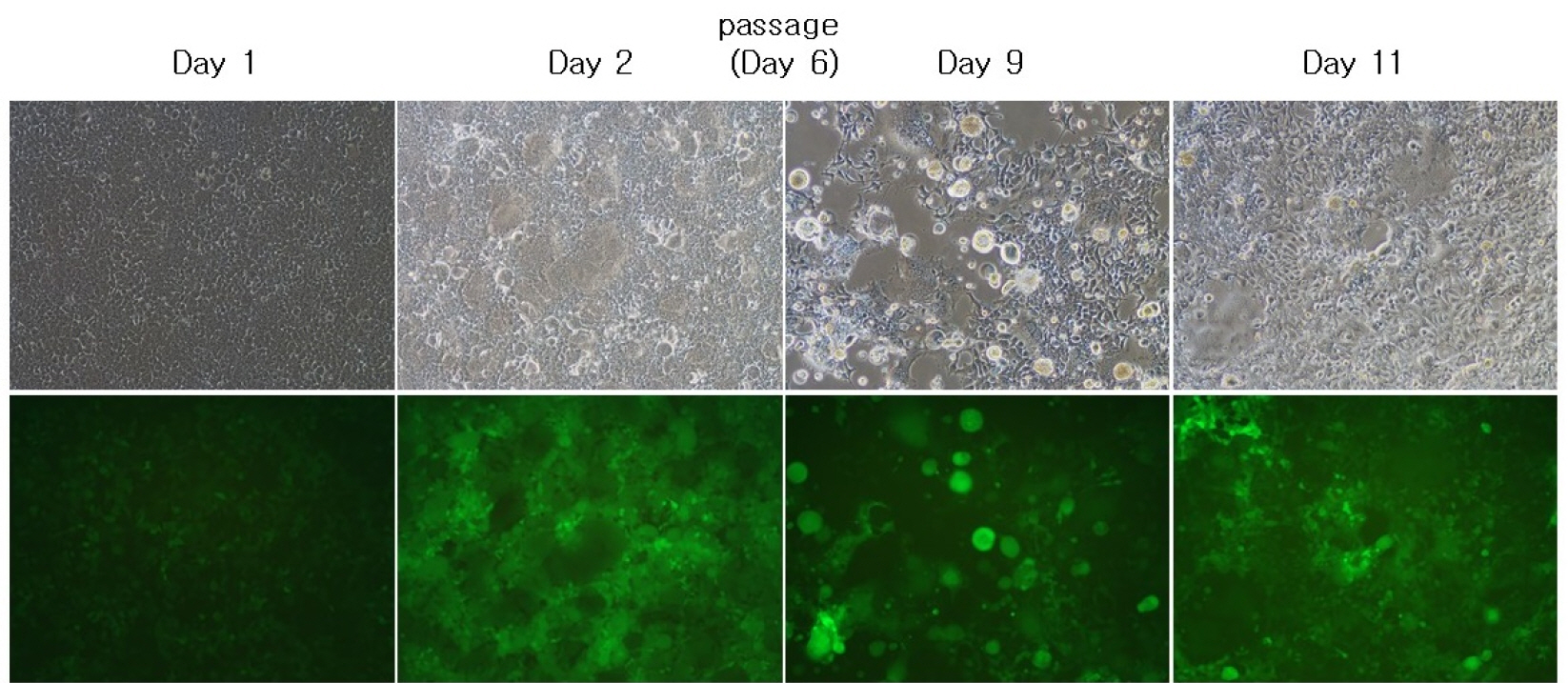
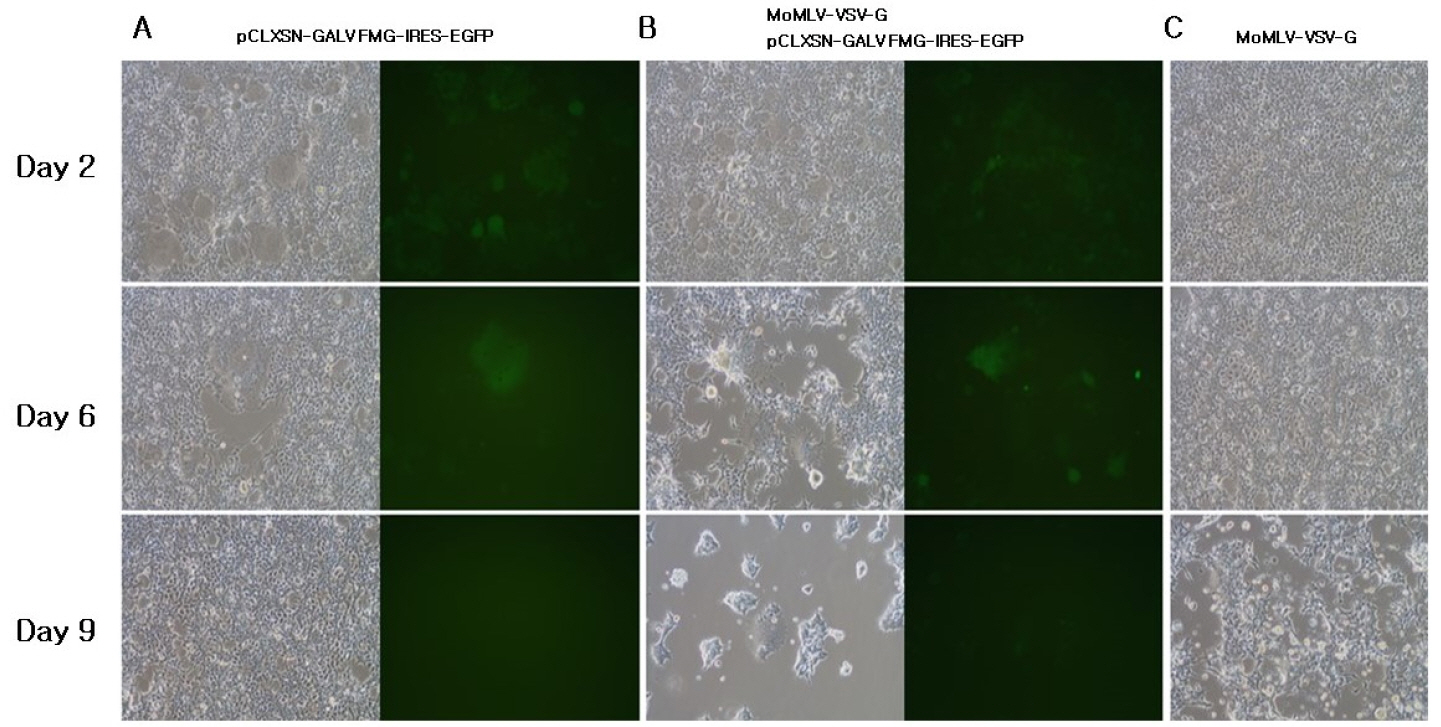
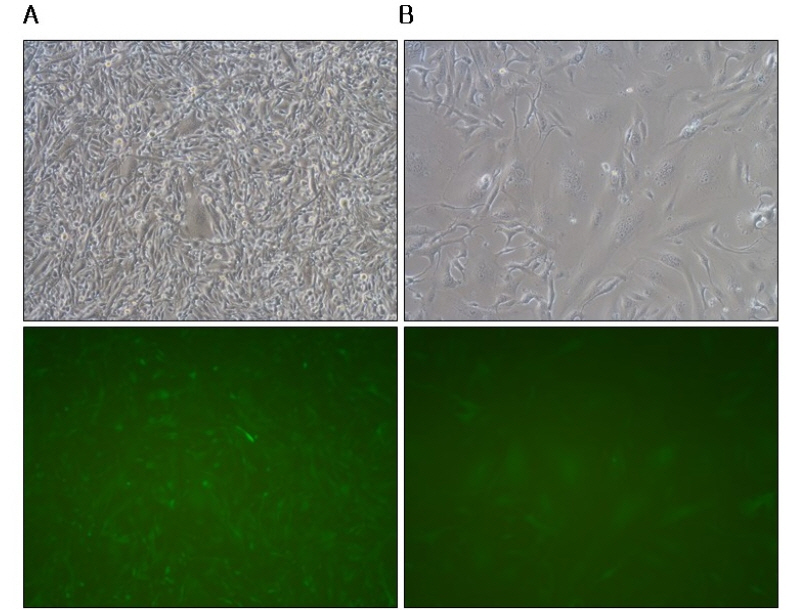
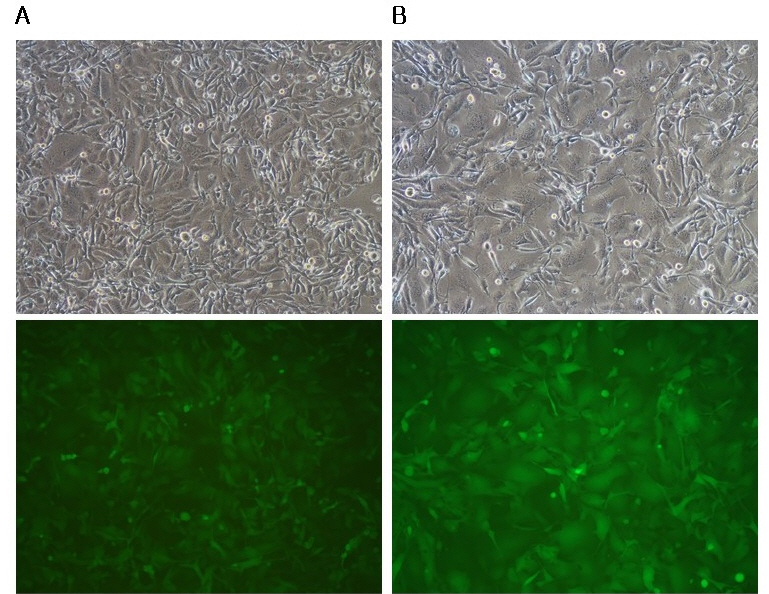
- A semi-replication-competent retroviral (s-RCR) vector system in which the gag-pol and env (GALV FMG, gibbon ape leukemia virus fusogenic membrane glycoprotein) genes were split into two separate packageable vectors was developed. These vectors are more efficient than replication-defective retroviral (RDR) vectors in gene delivery and have a higher transgene capacity than replication-competent retroviral (RCR) vectors. For thegag-pol vector construction, internal ribosomal entry site-enhanced green fluorescent protein (IRES-EGFP) was introduced downstream of the gag-pol sequence of the previously constructed MoMLV-10A1-EGFP vector to generate MoMLV-gag-pol-IRES-EGFP. For env vector construction, GALV FMG was inserted into the pCLXSN vector to generate pCLXSN-GALV FMG-IRES-EGFP.MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV FMG-IRES-EGFP were co-transfected into 293T cells to generate s-RCR viruses. These viruses propagated EGFP and induced syncytium formation due to the cytotoxicity of GALV FMG. To improve the cytotoxicity of s-RCR vector system, GALV FMG or the fusogenic envelope G glycoprotein of the vesicular stomatitis virus (VSV-G) was inserted into gag-pol vector. Co-transfection of MoMLV-gag-pol-IRES-GALV FMG + MoMLV-EGFP or MoMLV-VSV-G + pCLXSN-GALV FMG-IRES-EGFP in 293T cells induced stronger syncytium formation than s-RCR vectors (MoMLV-gag-pol-IRES-EGFP + pCLXSN-GALV FMG-IRES-EGFP). In addition, s-RCR stocks collected from transfected 293T cells induced syncytium formation in the human cancer cell lines HT1080 and TE671.Hence, the s-RCR vector systems developed in this study are useful tools for cancer gene therapy.
Figure
Reference
-
1. Hiraoka K, Kimura T, Logg CR, Tai CK, Haga K, Lawson GW, et al. Therapeutic efficacy of replication-competent retrovirus vector-mediated suicide gene therapy in a multifocal colorectal cancer metastasis model. Cancer Res 2007;67:5345-53.DOI: 10.1158/0008-5472.CAN-06-4673. PMID: 17545615.2. Kubo S, Takagi-Kimura M, Logg CR, Kasahara N. Highly efficient tumor transduction and antitumor efficacy in experimental human malignant mesothelioma using replicating gibbon ape leukemia virus. Cancer Gene Ther 2013;20:671-7.DOI: 10.1038/cgt.2013.67. PMID: 24201868.3. Kubo S, Takagi-Kimura M, Kasahara N. Efficient tumor transduction and antitumor efficacy in experimental human osteosarcoma using retroviral replicating vectors. Cancer Gene Ther 2019;26:41-7.DOI: 10.1038/s41417-018-0037-y. PMID: 30042500. PMCID: PMC6760559.4. Lu YC, Chen YJ, Yu YR, Lai YH, Cheng JC, Li YF, et al. Replicating retroviral vectors for oncolytic virotherapy of experimental hepatocellular carcinoma. Oncol Rep 2012;28:21-6.5. Solly SK, Trajcevski S, Frisén C, Holzer GW, Nelson E, Clerc B, et al. Replicative retroviral vectors for cancer gene therapy. Cancer Gene Ther 2003;10:30-9.DOI: 10.1038/sj.cgt.7700521. PMID: 12489026.6. Song JJ, Kim JH, Lee H, Kim E, Kim J, Park YS, et al. Enhancement of gene transfer efficiency into human cancer cells by modification of retroviral vectors and addition of chemicals. Oncol Rep 2000;7:119-24.DOI: 10.3892/or.7.1.119. PMID: 10601604.7. Tai CK, Wang WJ, Chen TC, Kasahara N. Single-shot, multicycle suicide gene therapy by replication-competent retrovirus vectors achieves long-term survival benefit in experimental glioma. Mol Ther 2005;12:842-51.DOI: 10.1016/j.ymthe.2005.03.017. PMID: 16257382.8. Qiao J, Moreno J, Sanchez-Perez L, Kottke T, Thompson J, Caruso M, et al. VSV-G pseudotyped, MuLV-based, semi-replication-competent retrovirus for cancer treatment. Gene Ther 2006;13:1457-70.DOI: 10.1038/sj.gt.3302782. PMID: 16724095.9. Trajcevski S, Solly SK, Frisén C, Trenado A, Cosset FL, Klatzmann D. Characterization of a semi-replicative gene delivery system allowing propagation of complementary defective retroviral vectors. J Gene Med 2005;7:276-87.DOI: 10.1002/jgm.663. PMID: 15515136.10. Bateman A, Bullough F, Murphy S, Emiliusen L, Lavillette D, Cosset FL, et al. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res 2000;60:1492-7.11. Fielding AK, Chapel-Fernandes S, Chadwick MP, Bullough FJ, Cosset FL, Russell SJ. A hyperfusogenic gibbon ape leukemia envelope glycoprotein: targeting of a cytotoxic gene by ligand display. Hum Gene Ther 2000;11:817-26.DOI: 10.1089/10430340050015437. PMID: 10779159.12. Fu X, Tao L, Jin A, Vile R, Brenner MK, Zhang X. Expression of a fusogenic membrane glycoprotein by an oncolytic herpes simplex virus potentiates the viral antitumor effect. Mol Ther 2003;7:748-54.DOI: 10.1016/S1525-0016(03)00092-3.13. Guedan S, Grases D, Rojas JJ, Gros A, Vilardell F, Vile R, et al. GALV expression enhances the therapeutic efficacy of an oncolytic adenovirus by inducing cell fusion and enhancing virus distribution. Gene Ther 2012;19:1048-57.DOI: 10.1038/gt.2011.184. PMID: 22113313.14. Galanis E, Bateman A, Johnson K, Diaz RM, James CD, Vile R, et al. Use of viral fusogenic membrane glycoproteins as novel therapeutic transgenes in gliomas. Hum Gene Ther 2001;12:811-21.DOI: 10.1089/104303401750148766. PMID: 11339897.15. Lin EH, Salon C, Brambilla E, Lavillette D, Szecsi J, Cosset FL, et al. Fusogenic membrane glycoproteins induce syncytia formation and death in vitro and in vivo: a potential therapy agent for lung cancer. Cancer Gene Ther 2010;17:256-65.DOI: 10.1038/cgt.2009.74. PMID: 19893593.16. Zhang J, Frolov I, Russell SJ. Gene therapy for malignant glioma using Sindbis vectors expressing a fusogenic membrane glycoprotein. J Gene Med 2004;6:1082-91.DOI: 10.1002/jgm.605. PMID: 15368589.17. Chung M, Kizhatil K, Albritton LM, Gaulton GN. Induction of syncytia by neuropathogenic murine leukemia viruses depends on receptor density, host cell determinants, and the intrinsic fusion potential of envelope protein. J Virol 1999;73:9377-85.DOI: 10.1128/JVI.73.11.9377-9385.1999. PMID: 10516046. PMCID: PMC112972.18. Diaz RM, Bateman A, Emiliusen L, Fielding A, Trono D, Russell SJ, et al. A lentiviral vector expressing a fusogenic glycoprotein for cancer gene therapy. Gene Ther 2000;7:1656-63.DOI: 10.1038/sj.gt.3301277. PMID: 11083474.19. Ferlin A, Raux H, Baquero E, Lepault J, Gaudin Y. Characterization of pH-sensitive molecular switches that trigger the structural transition of vesicular stomatitis virus glycoprotein from the postfusion state toward the prefusion state. J Virol 2014;88:13396-409.DOI: 10.1128/JVI.01962-14. PMID: 25210175. PMCID: PMC4249061.20. Rücker P, Wieninger SA, Ullmann GM, Sticht H. pH-dependent molecular dynamics of vesicular stomatitis virus glycoprotein G. Proteins 2012;80:2601-13.DOI: 10.1002/prot.24145. PMID: 22806964.21. Yao Y, Ghosh K, Epand RF, Epand RM, Ghosh HP. Membrane fusion activity of vesicular stomatitis virus glycoprotein G is induced by low pH but not by heat or denaturant. Virology 2003;310:319-32.DOI: 10.1016/S0042-6822(03)00146-6.22. Ragheb JA, Anderson WF. pH-independent murine leukemia virus ecotropic envelope-mediated cell fusion: implications for the role of the R peptide and p12E TM in viral entry. J Virol 1994;68:3220-31. DOI: 10.1128/JVI.68.5.3220-3231.1994. PMID: 8151784.23. Kavanaugh MP, Miller DG, Zhang W, Law W, Kozak SL, Kabat D, et al. Cell-surface receptors for gibbon ape leukemia virus and amphotropic murine retrovirus are inducible sodium-dependent phosphate symporters. Proc Natl Acad Sci U S A 1994;91:7071-5.DOI: 10.1073/pnas.91.15.7071. PMID: 8041748. PMCID: PMC44340.24. Lam JS, Reeves ME, Cowherd R, Rosenberg SA, Hwu P. Improved gene transfer into human lymphocytes using retroviruses with the gibbon ape leukemia virus envelope. Hum Gene Ther 1996;7:1415-22.DOI: 10.1089/hum.1996.7.12-1415. PMID: 8844200.25. Miller AD. Cell-surface receptors for retroviruses and implications for gene transfer. Proc Natl Acad Sci U S A 1996;93:11407-13.DOI: 10.1073/pnas.93.21.11407. PMID: 8876148. PMCID: PMC38070.26. Jin SY, Jung YT. Construction of a replication-competent retroviral vector for expression of the VSV-G envelope glycoprotein for cancer gene therapy. Arch Virol 2020;165:1089-97.DOI: 10.1007/s00705-020-04585-8. PMID: 32146506.27. Christodoulopoulos I, Cannon PM. Sequences in the cytoplasmic tail of the gibbon ape leukemia virus envelope protein that prevent its incorporation into lentivirus vectors. J Virol 2001;75:4129-38.DOI: 10.1128/JVI.75.9.4129-4138.2001. PMID: 11287562. PMCID: PMC114158.28. Tomás HA, Mestre DA, Rodrigues AF, Guerreiro MR, Carrondo MJT, Coroadinha AS. Improved GaLV-TR glycoproteins to pseudotype lentiviral vectors: impact of viral protease activity in the production of LV pseudotypes. Mol Ther Methods Clin 2019;15:1-8.DOI: 10.1016/j.omtm.2019.08.001. PMID: 31528654. PMCID: PMC6742969.29. Logg CR, Logg A, Tai CK, Cannon PM, Kasahara N. Genomic stability of murine leukemia viruses containing insertions at the Env-3’ untranslated region boundary. J Virol 2001;75:6989-98.DOI: 10.1128/JVI.75.15.6989-6998.2001. PMID: 11435579. PMCID: PMC114427.30. Nowrouzi A, Glimm H, von Kalle C, Schmidt M. Retroviral vectors: post entry events and genomic alterations. Viruses 2011;3:429-55.DOI: 10.3390/v3050429. PMID: 21994741. PMCID: PMC3185758.31. Mothes W, Sherer NM, Jin J, Zhong P. Virus cell-to-cell transmission. J Virol 2010;84:8360-8.DOI: 10.1128/JVI.00443-10. PMID: 20375157. PMCID: PMC2918988.32. Sattentau QJ. Cell-to-Cell Spread of Retroviruses. Viruses 2010;2:1306-21.DOI: 10.3390/v2061306. PMID: 21994681. PMCID: PMC3185708.33. Lee H, Song JJ, Kim E, Yun CO, Choi J, Lee B, et al. Efficient gene transfer of VSV-G pseudotyped retroviral vector to human brain tumor. Gene Ther 2001;8:268-73.DOI: 10.1038/sj.gt.3301390. PMID: 11313800.
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- A simple human immunodeficiency virus vector system for selective infection of CD4(+) cells and inducible expression of foreign genes
- Comparison of Efficiency of Infection of Human Cancer Cell Lines Via Retroviral Vector System
- Probing the Utility of Vascular Smooth Muscle Cells as a Target Cell for ex vivo Cardiovascular Gene Therapy
- Identification of Retroviral Vectors Producing High Viral Titer
- Vesicular Stomatitis Virus G Glycoprotein and ATRA Enhanced Bystander Killing of Chemoresistant Leukemic Cells by Herpes Simplex Virus Thymidine Kinase/Ganciclovir