J Bacteriol Virol.
2020 Sep;50(3):158-167. 10.4167/jbv.2020.50.3.158.
Overview of the Efficacy of Human Papillomavirus Virus Vaccines
- Affiliations
-
- 1Division of Viral Disease Research, Center for Infectious Disease Research, Korea National Institute of Health, Chungbuk 28159, Republic of Korea
- KMID: 2512134
- DOI: http://doi.org/10.4167/jbv.2020.50.3.158
Abstract
- Human papillomavirus (HPV) infection is the main cause of cervical cancer and major viruses related to carcinogenesis in various malignant diseases such as cervical cancer, vaginal cancer, vulvar cancer, anal cancer, and head and neck cancer. Cervical cancer is the second most prevalent female cancer in the world and the fourth in Korea. Prophylactic HPV vaccines in widespread use include the used in South Korea to prevent cervical cancer are bivalent (2-valent HPV vaccine; Cervarix), quadrivalent (4-valent HPV vaccine; Gardasil), and nonavalent (9-valent HPV vaccine; Gardasil9). Since HPV vaccines the first approval in 2006, 115 countries have include HPV vaccines in their national immunization programs, that its preventive effect is as much as 70%, and that the incidence of high-risk types of HPV has gradually decreased. According to HPV cohort studies in Korea, about 26% of adult women have an HPV vaccination history and show a low incidence of HPV-16/18 genotypes compared to unvaccinated women. In the countries that National Immunization Programs for HPV vaccine were conducted earlier than in Korea, the safety, efficacy, and effectiveness of HPV vaccines have been reported. Therefore, it is considered that basic research including an analysis of the effectiveness of HPV vaccines for policy decisions related to the expanding the HPV vaccine coverage and introducing of new vaccine in the future.
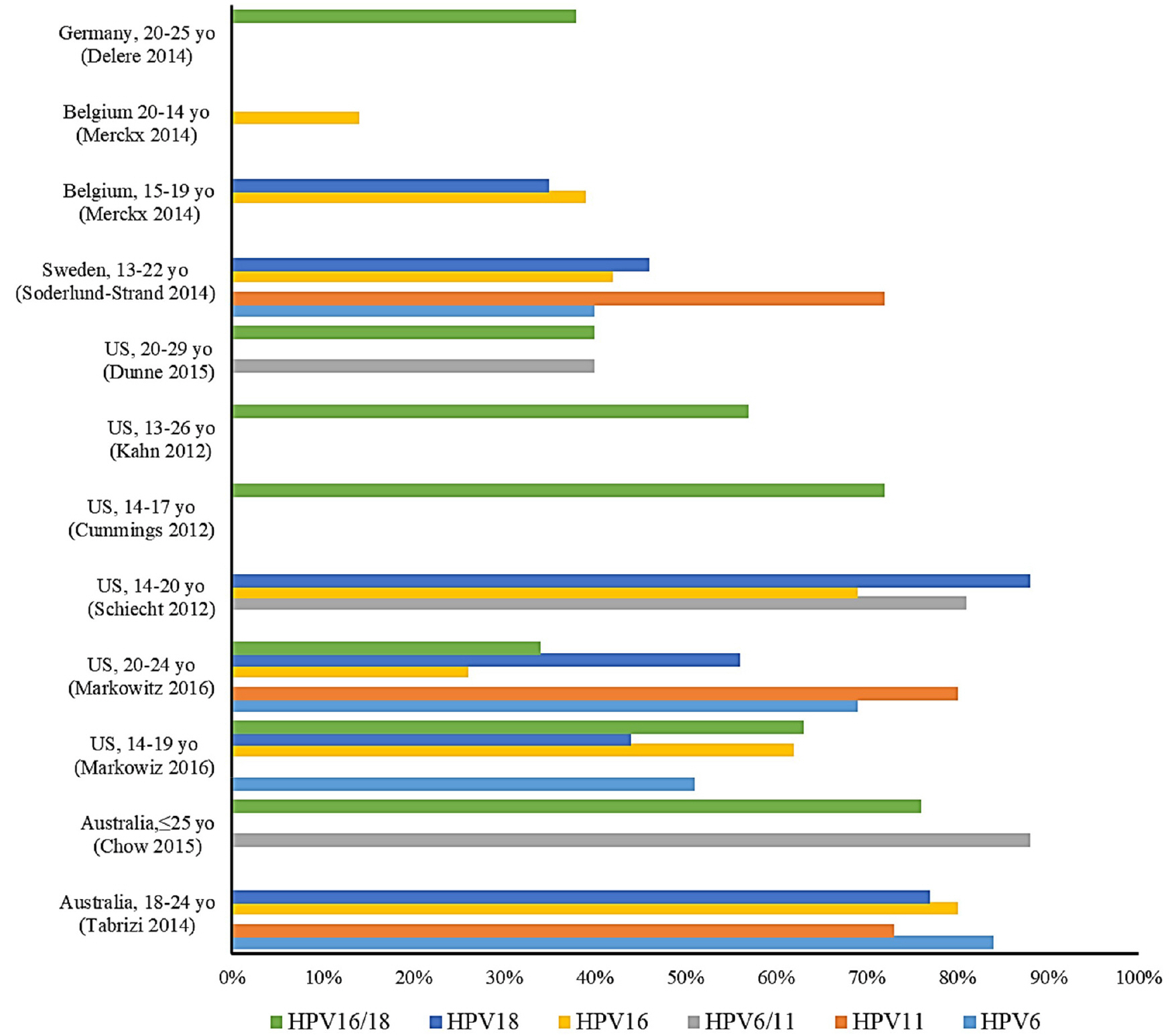
Figure
Cited by 1 articles
-
Prevalence and Treatment of Vulvar Cancer From 2014−2018: A Nationwide Population-Based Study in Korea
Yung-Taek Ouh, Dongwoo Kang, Hoseob Kim, Jae Kwan Lee, Jin Hwa Hong
J Korean Med Sci. 2022;37(4):e25. doi: 10.3346/jkms.2022.37.e25.
Reference
-
1. Seong J, Ryou S, Yoo M, Lee J, Kim K, Jee Y, et al. Status of HPV vaccination among HPV-infected women aged 20-60 years with abnormal cervical cytology in South Korea: a multicenter, retrospective study. J Gynecol Oncol 2020;31:e4.DOI: 10.3802/jgo.2020.31.e4. PMID: 31788994. PMCID: PMC6918886.2. Kim G, Kim J, Song R. Study on the Expansion of HPV Vaccination for the Korean National Immunization Program. Public Health Weekly Report (KCDC). 2019;12.3. Kim MA, Han GH, Kim JH, Seo K. Current Status of Human Papillomavirus Infection and Introduction of Vaccination to the National Immunization Program in Korea: an Overview. J Korean Med Sci 2018;33:e331.DOI: 10.3346/jkms.2018.33.e331. PMID: 30584412. PMCID: PMC6300657.4. Pinto LA, Dillner J, Beddows S, Unger ER. Immunogenicity of HPV prophylactic vaccines: Serology assays and their use in HPV vaccine evaluation and development. Vaccine 2018;36:4792-9.DOI: 10.1016/j.vaccine.2017.11.089. PMID: 29361344. PMCID: PMC6050153.5. Stanley M. Immunobiology of HPV and HPV vaccines. Gynecol Oncol 2008;109:S15-21.DOI: 10.1016/j.ygyno.2008.02.003. PMID: 18474288.6. Bruni L. Global vaccine uptake and projected cervical cancer disease reductions. HPV World 2020;1:4.7. Brotherton JML. Impact of HPV vaccination: Achievements and future challenges. Papillomavirus Res 2019;7:138-40.DOI: 10.1016/j.pvr.2019.04.004. PMID: 30978413. PMCID: PMC6465571.8. McGregor S, Saulo D, Brotherton JML, Liu B, Phillips S, Skinner SR, et al. Decline in prevalence of human papillomavirus infection following vaccination among Australian Indigenous women, a population at higher risk of cervical cancer: The VIP-I study. Vaccine 2018;36:4311-6.DOI: 10.1016/j.vaccine.2018.05.104. PMID: 29880245.9. McClung NM, Gargano JW, Bennett NM, Niccolai LM, Abdullah N, Griffin MR, et al. Trends in Human Papillomavirus Vaccine Types 16 and 18 in Cervical Precancers, 2008-2014. Cancer Epidemiol Biomarkers Prev 2019;28:602-9.DOI: 10.1158/1055-9965.EPI-18-0885. PMID: 30792242. PMCID: PMC6526945.10. Steben M, Tan Thompson M, Rodier C, Mallette N, Racovitan V, DeAngelis F, et al. A Review of the Impact and Effectiveness of the Quadrivalent Human Papillomavirus Vaccine: 10 Years of Clinical Experience in Canada. J Obstet Gynaecol Can. 2018;40:1635-45.DOI: 10.1016/j.jogc.2018.05.024. PMID: 30341021.11. Heard I, Tondeur L, Arowas L, Demazoin M, Falguières M, Parent Du Chatelet I, et al. Effectiveness of Human Papillomavirus Vaccination on Prevalence of Vaccine Genotypes in Young Sexually Active Women in France. J Infect Dis 2017;215:757-63.DOI: 10.1093/infdis/jiw639. PMID: 28011911.12. Mehanna H, Bryant TS, Babrah J, Louie K, Bryant JL, Spruce RJ, et al. Human Papillomavirus (HPV) Vaccine Effectiveness and Potential Herd Immunity for Reducing Oncogenic Oropharyngeal HPV-16 Prevalence in the United Kingdom: A Cross-sectional Study. Clin Infect Dis 2019;69:1296-302.DOI: 10.1093/cid/ciy1081. PMID: 30590469. PMCID: PMC6763631.13. Sonnenberg P, Tanton C, Mesher D, King E, Beddows S, Field N, et al. Epidemiology of genital warts in the British population: implications for HPV vaccination programmes. Sex Transm Infect 2019;95:386-90.DOI: 10.1136/sextrans-2018-053786. PMID: 30723185. PMCID: PMC6678036.14. Patel C, Brotherton JM, Pillsbury A, Jayasinghe S, Donovan B, Macartney K, et al. The impact of 10 years of human papillomavirus (HPV) vaccination in Australia: what additional disease burden will a nonavalent vaccine prevent?. Euro Surveill 2018;23:1700737.DOI: 10.2807/1560-7917.ES.2018.23.41.1700737. PMID: 30326995. PMCID: PMC6194907.15. Karube A, Saito F, Nakamura E, Shitara A, Ono N, Konno M, et al. Reduction in HPV 16/18 prevalence among young women following HPV vaccine introduction in a highly vaccinated district, Japan, 2008-2017. J Rural Med 2019;14:48-57.DOI: 10.2185/jrm.2986. PMID: 31191766. PMCID: PMC6545435.