Endocrinol Metab.
2020 Dec;35(4):756-764. 10.3803/EnM.2020.402.
Coordination of Multiple Cellular Processes by NR5A1/Nr5a1
- Affiliations
-
- 1Division of Biology of Sex Differences, Graduate School of Medical Sciences, and Graduate School of Systems Life Sciences, Kyushu University, Fukuoka, Japan
- KMID: 2511002
- DOI: http://doi.org/10.3803/EnM.2020.402
Abstract
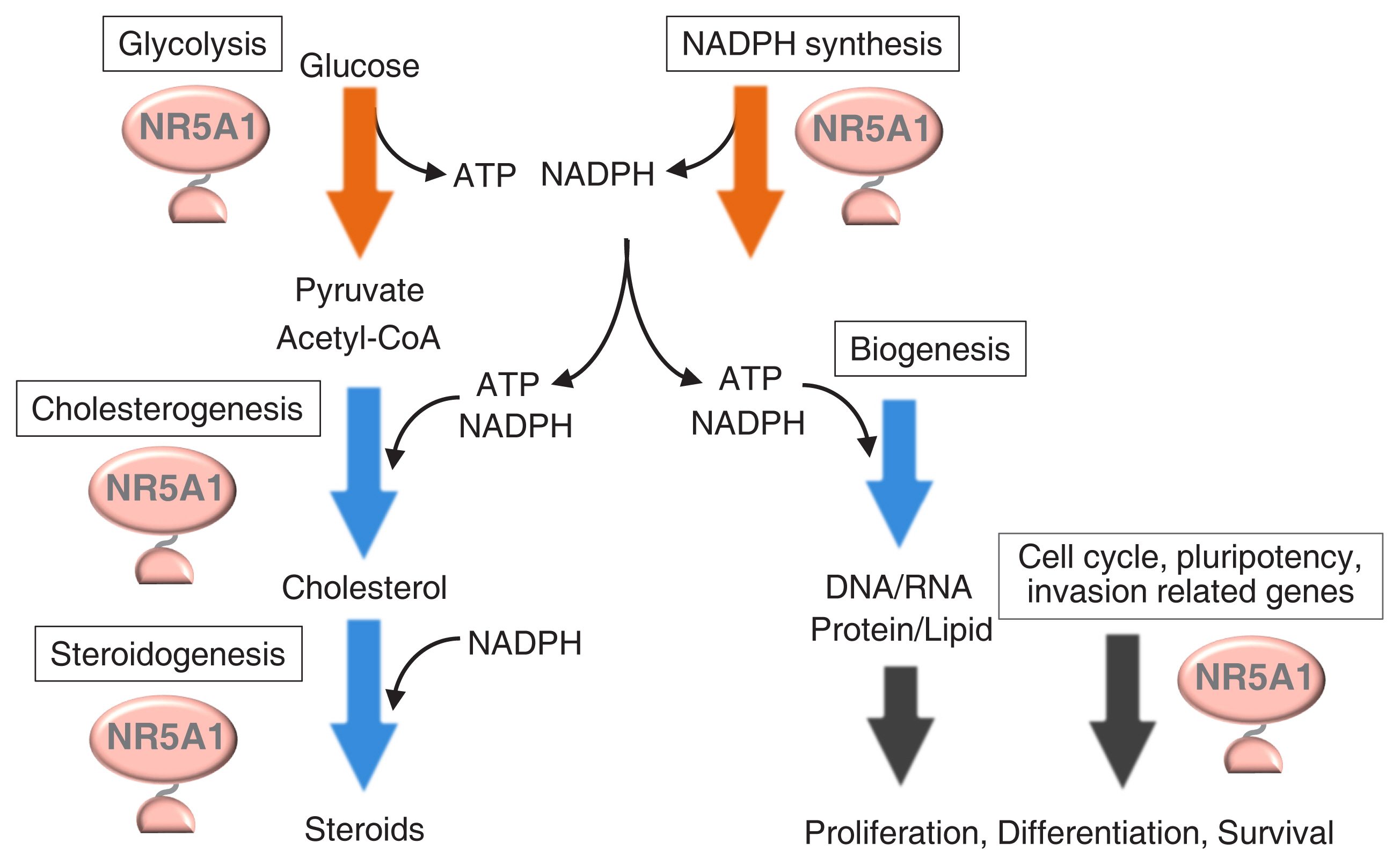
- The agenesis of the gonads and adrenal gland in revealed by knockout mouse studies strongly suggested a crucial role for Nr5a1 (SF-1 or Ad4BP) in organ development. In relation to these striking phenotypes, NR5A1/Nr5a1 has the potential to reprogram cells to steroidogenic cells, endow pluripotency, and regulate cell proliferation. However, due to limited knowledge regarding NR5A1 target genes, the mechanism by which NR5A1/Nr5a1 regulates these fundamental processes has remained unknown. Recently, newlyestablished technologies have enabled the identification of NR5A1 target genes related to multiple metabolic processes, as well as the aforementioned biological processes. Considering that active cellular processes are expected to be accompanied by active metabolism, NR5A1 may act as a key factor for processes such as cell differentiation, proliferation, and survival by coordinating these processes with cellular metabolism. A complete and definite picture of the cellular processes coordinated by NR5A1/Nr5a1 could be depicted by accumulating evidence of the potential target genes through whole genome studies.
Keyword
Figure
Cited by 1 articles
-
Subunit-Specific Developmental Roles of PI3K in SF1-Expressing Cells
My Khanh Q. Huynh, Sang Hee Lyoo, Dong Joo Yang, Yun-Hee Choi, Ki Woo Kim
Endocrinol Metab. 2024;39(5):793-802. doi: 10.3803/EnM.2024.1999.
Reference
-
1. Lala DS, Rice DA, Parker KL. Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu-factor I. Mol Endocrinol. 1992; 6:1249–58.
Article2. Morohashi K, Honda S, Inomata Y, Handa H, Omura T. A common trans-acting factor, Ad4-binding protein, to the promoters of steroidogenic P-450s. J Biol Chem. 1992; 267:17913–9.
Article3. Honda S, Morohashi K, Nomura M, Takeya H, Kitajima M, Omura T. Ad4BP regulating steroidogenic P-450 gene is a member of steroid hormone receptor superfamily. J Biol Chem. 1993; 268:7494–502.
Article4. Morohashi Ki. Gonadal and extragonadal functions of Ad4BP/SF-1: developmental aspects. Trends Endocrinol Metab. 1999; 10:169–73.
Article5. Parker KL, Schimmer BP. Steroidogenic factor 1: a key determinant of endocrine development and function. Endocr Rev. 1997; 18:361–77.
Article6. Hu MC, Hsu NC, Pai CI, Wang CK, Chung BC. Functions of the upstream and proximal steroidogenic factor 1 (SF-1)-binding sites in the CYP11A1 promoter in basal transcription and hormonal response. Mol Endocrinol. 2001; 15:812–8.
Article7. Shih MC, Hsu NC, Huang CC, Wu TS, Lai PY, Chung BC. Mutation of mouse Cyp11a1 promoter caused tissue-specific reduction of gene expression and blunted stress response without affecting reproduction. Mol Endocrinol. 2008; 22:915–23.8. Luo X, Ikeda Y, Parker KL. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994; 77:481–90.
Article9. Sadovsky Y, Crawford PA, Woodson KG, Polish JA, Clements MA, Tourtellotte LM, et al. Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 side-chain-cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc Natl Acad Sci U S A. 1995; 92:10939–43.
Article10. Morohashi KI, Omura T. Ad4BP/SF-1, a transcription factor essential for the transcription of steroidogenic cytochrome P450 genes and for the establishment of the reproductive function. FASEB J. 1996; 10:1569–77.
Article11. Ingraham HA, Lala DS, Ikeda Y, Luo X, Shen WH, Nachtigal MW, et al. The nuclear receptor steroidogenic factor 1 acts at multiple levels of the reproductive axis. Genes Dev. 1994; 8:2302–12.
Article12. Shinoda K, Lei H, Yoshii H, Nomura M, Nagano M, Shiba H, et al. Developmental defects of the ventromedial hypothalamic nucleus and pituitary gonadotroph in the Ftz-F1 disrupted mice. Dev Dyn. 1995; 204:22–9.
Article13. Ikeda Y, Luo X, Abbud R, Nilson JH, Parker KL. The nuclear receptor steroidogenic factor 1 is essential for the formation of the ventromedial hypothalamic nucleus. Mol Endocrinol. 1995; 9:478–86.
Article14. Morohashi K, Tsuboi-Asai H, Matsushita S, Suda M, Nakashima M, Sasano H, et al. Structural and functional abnormalities in the spleen of an mFtz-F1 gene-disrupted mouse. Blood. 1999; 93:1586–94.15. Zhao L, Bakke M, Parker KL. Pituitary-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2001; 185:27–32.
Article16. Segal JP, Stallings NR, Lee CE, Zhao L, Socci N, Viale A, et al. Use of laser-capture microdissection for the identification of marker genes for the ventromedial hypothalamic nucleus. J Neurosci. 2005; 25:4181–8.
Article17. Tran PV, Akana SF, Malkovska I, Dallman MF, Parada LF, Ingraham HA. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J Comp Neurol. 2006; 498:637–48.18. Kim KW, Zhao L, Parker KL. Central nervous system-specific knockout of steroidogenic factor 1. Mol Cell Endocrinol. 2009; 300:132–6.
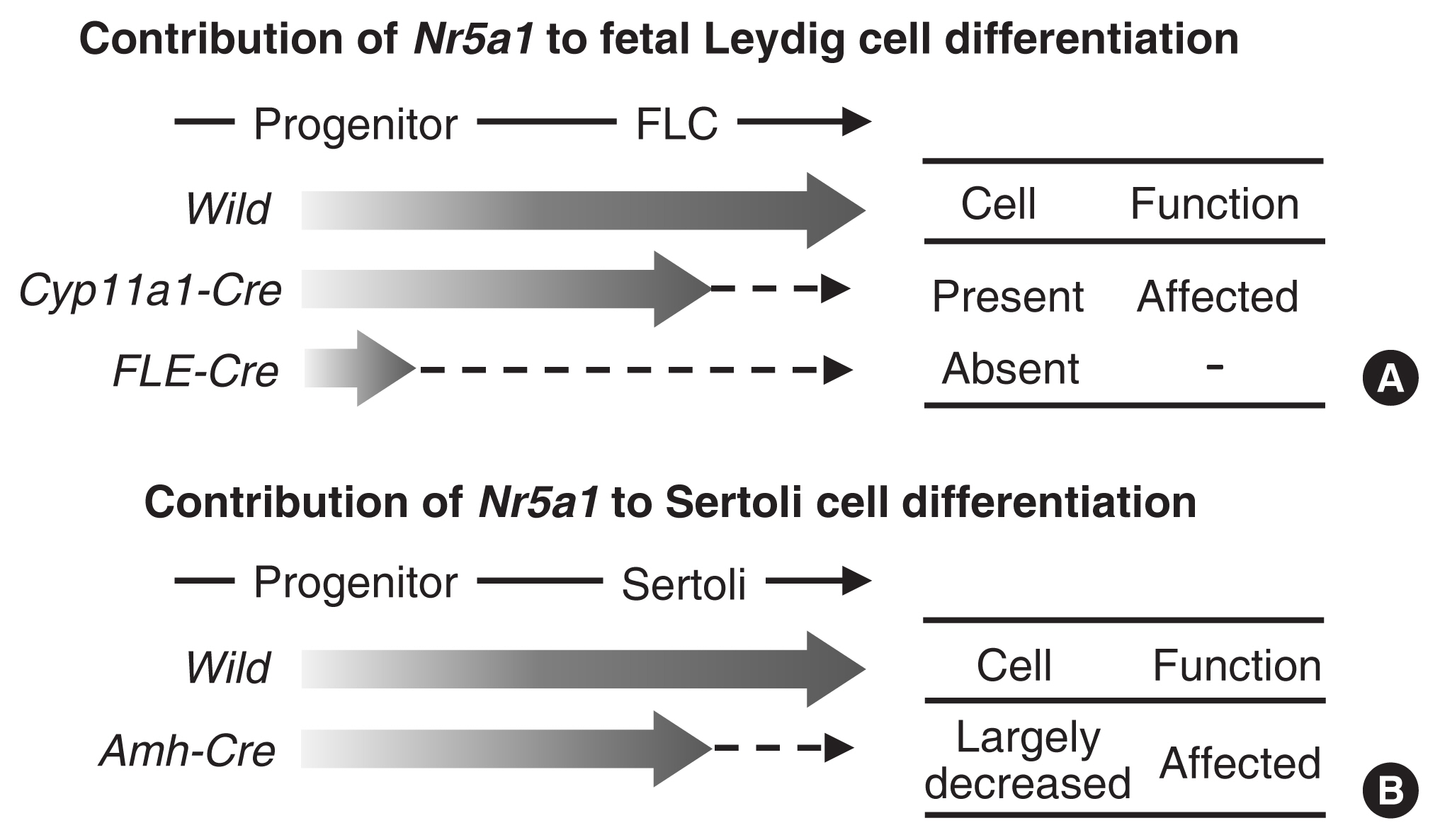
Article19. Jeyasuria P, Ikeda Y, Jamin SP, Zhao L, De Rooij DG, Themmen AP, et al. Cell-specific knockout of steroidogenic factor 1 reveals its essential roles in gonadal function. Mol Endocrinol. 2004; 18:1610–9.
Article20. Pelusi C, Ikeda Y, Zubair M, Parker KL. Impaired follicle development and infertility in female mice lacking steroidogenic factor 1 in ovarian granulosa cells. Biol Reprod. 2008; 79:1074–83.21. Buaas FW, Gardiner JR, Clayton S, Val P, Swain A. In vivo evidence for the crucial role of SF1 in steroid-producing cells of the testis, ovary and adrenal gland. Development. 2012; 139:4561–70.
Article22. Shima Y, Miyabayashi K, Sato T, Suyama M, Ohkawa Y, Doi M, et al. Fetal Leydig cells dedifferentiate and serve as adult Leydig stem cells. Development. 2018; 145:dev169136.
Article23. Anamthathmakula P, Miryala CSJ, Moreci RS, Kyathanahalli C, Hassan SS, Condon JC, et al. Steroidogenic factor 1 (Nr5a1) is required for sertoli cell survival post sex determination. Sci Rep. 2019; 9:4452.
Article24. Miyabayashi K, Katoh-Fukui Y, Ogawa H, Baba T, Shima Y, Sugiyama N, et al. Aristaless related homeobox gene, Arx, is implicated in mouse fetal Leydig cell differentiation possibly through expressing in the progenitor cells. PLoS One. 2013; 8:e68050.
Article25. De Santa Barbara P, Bonneaud N, Boizet B, Desclozeaux M, Moniot B, Sudbeck P, et al. Direct interaction of SRY-related protein SOX9 and steroidogenic factor 1 regulates transcription of the human anti-Müllerian hormone gene. Mol Cell Biol. 1998; 18:6653–65.
Article26. Tremblay JJ, Viger RS. Transcription factor GATA-4 enhances Müllerian inhibiting substance gene transcription through a direct interaction with the nuclear receptor SF-1. Mol Endocrinol. 1999; 13:1388–401.27. Watanabe K, Clarke TR, Lane AH, Wang X, Donahoe PK. Endogenous expression of Müllerian inhibiting substance in early postnatal rat sertoli cells requires multiple steroidogenic factor-1 and GATA-4-binding sites. Proc Natl Acad Sci U S A. 2000; 97:1624–9.
Article28. Lasala C, Carre-Eusebe D, Picard JY, Rey R. Subcellular and molecular mechanisms regulating anti-Müllerian hormone gene expression in mammalian and nonmammalian species. DNA Cell Biol. 2004; 23:572–85.
Article29. Crawford PA, Sadovsky Y, Milbrandt J. Nuclear receptor steroidogenic factor 1 directs embryonic stem cells toward the steroidogenic lineage. Mol Cell Biol. 1997; 17:3997–4006.
Article30. Gondo S, Yanase T, Okabe T, Tanaka T, Morinaga H, Nomura M, et al. SF-1/Ad4BP transforms primary long-term cultured bone marrow cells into ACTH-responsive steroidogenic cells. Genes Cells. 2004; 9:1239–47.
Article31. Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M, et al. Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology. 2006; 147:4104–11.
Article32. Rotgers E, Jorgensen A, Yao HH. At the crossroads of fate-somatic cell lineage specification in the fetal gonad. Endocr Rev. 2018; 39:739–59.
Article33. Buganim Y, Itskovich E, Hu YC, Cheng AW, Ganz K, Sarkar S, et al. Direct reprogramming of fibroblasts into embryonic Sertoli-like cells by defined factors. Cell Stem Cell. 2012; 11:373–86.
Article34. Liang J, Wang N, He J, Du J, Guo Y, Li L, et al. Induction of Sertoli-like cells from human fibroblasts by NR5A1 and GATA4. Elife. 2019; 8:e48767.
Article35. Yang Y, Li Z, Wu X, Chen H, Xu W, Xiang Q, et al. Direct reprogramming of mouse fibroblasts toward Leydig-like cells by defined factors. Stem Cell Reports. 2017; 8:39–53.
Article36. Fayard E, Auwerx J, Schoonjans K. LRH-1: an orphan nuclear receptor involved in development, metabolism and steroidogenesis. Trends Cell Biol. 2004; 14:250–60.
Article37. Saxena D, Escamilla-Hernandez R, Little-Ihrig L, Zeleznik AJ. Liver receptor homolog-1 and steroidogenic factor-1 have similar actions on rat granulosa cell steroidogenesis. Endocrinology. 2007; 148:726–34.
Article38. Gu P, Goodwin B, Chung AC, Xu X, Wheeler DA, Price RR, et al. Orphan nuclear receptor LRH-1 is required to maintain Oct4 expression at the epiblast stage of embryonic development. Mol Cell Biol. 2005; 25:3492–505.
Article39. Heng JC, Feng B, Han J, Jiang J, Kraus P, Ng JH, et al. The nuclear receptor Nr5a2 can replace Oct4 in the reprogramming of murine somatic cells to pluripotent cells. Cell Stem Cell. 2010; 6:167–74.
Article40. Yamauchi K, Ikeda T, Hosokawa M, Nakatsuji N, Kawase E, Chuma S, et al. Overexpression of nuclear receptor 5a1 induces and maintains an intermediate state of conversion between primed and naive pluripotency. Stem Cell Reports. 2020; 14:506–19.
Article41. Bland ML, Jamieson CA, Akana SF, Bornstein SR, Eisenhofer G, Dallman MF, et al. Haploinsufficiency of steroidogenic factor-1 in mice disrupts adrenal development leading to an impaired stress response. Proc Natl Acad Sci U S A. 2000; 97:14488–93.42. Beuschlein F, Mutch C, Bavers DL, Ulrich-Lai YM, Engeland WC, Keegan C, et al. Steroidogenic factor-1 is essential for compensatory adrenal growth following unilateral adrenalectomy. Endocrinology. 2002; 143:3122–35.
Article43. Zubair M, Oka S, Parker KL, Morohashi K. Transgenic expression of Ad4BP/SF-1 in fetal adrenal progenitor cells leads to ectopic adrenal formation. Mol Endocrinol. 2009; 23:1657–67.44. Doghman M, Karpova T, Rodrigues GA, Arhatte M, De Moura J, Cavalli LR, et al. Increased steroidogenic factor-1 dosage triggers adrenocortical cell proliferation and cancer. Mol Endocrinol. 2007; 21:2968–87.
Article45. Doghman M, Cazareth J, Douguet D, Madoux F, Hodder P, Lalli E. Inhibition of adrenocortical carcinoma cell proliferation by steroidogenic factor-1 inverse agonists. J Clin Endocrinol Metab. 2009; 94:2178–83.
Article46. Ishimaru Y, Komatsu T, Kasahara M, Katoh-Fukui Y, Ogawa H, Toyama Y, et al. Mechanism of asymmetric ovarian development in chick embryos. Development. 2008; 135:677–85.
Article47. Syu JS, Baba T, Huang JY, Ogawa H, Hsieh CH, Hu JX, et al. Lysosomal activity maintains glycolysis and cyclin E1 expression by mediating Ad4BP/SF-1 stability for proper steroidogenic cell growth. Sci Rep. 2017; 7:240.
Article48. Lewis AE, Rusten M, Hoivik EA, Vikse EL, Hansson ML, Wallberg AE, et al. Phosphorylation of steroidogenic factor 1 is mediated by cyclin-dependent kinase 7. Mol Endocrinol. 2008; 22:91–104.
Article49. Fisher RP. Secrets of a double agent: CDK7 in cell-cycle control and transcription. J Cell Sci. 2005; 118:5171–80.
Article50. Lai PY, Wang CY, Chen WY, Kao YH, Tsai HM, Tachibana T, et al. Steroidogenic factor 1 (NR5A1) resides in centrosomes and maintains genomic stability by controlling centrosome homeostasis. Cell Death Differ. 2011; 18:1836–44.
Article51. Wang CY, Kao YH, Lai PY, Chen WY, Chung BC. Steroidogenic factor 1 (NR5A1) maintains centrosome homeostasis in steroidogenic cells by restricting centrosomal DNA-dependent protein kinase activation. Mol Cell Biol. 2013; 33:476–84.
Article52. Ferraz-de-Souza B, Lin L, Shah S, Jina N, Hubank M, Dattani MT, et al. ChIP-on-chip analysis reveals angiopoietin 2 (Ang2, ANGPT2) as a novel target of steroidogenic factor-1 (SF-1, NR5A1) in the human adrenal gland. FASEB J. 2011; 25:1166–75.
Article53. Ju Y, Mizutani T, Imamichi Y, Yazawa T, Matsumura T, Kawabe S, et al. Nuclear receptor 5A (NR5A) family regulates 5-aminolevulinic acid synthase 1 (ALAS1) gene expression in steroidogenic cells. Endocrinology. 2012; 153:5522–34.
Article54. Doghman M, Figueiredo BC, Volante M, Papotti M, Lalli E. Integrative analysis of SF-1 transcription factor dosage impact on genome-wide binding and gene expression regulation. Nucleic Acids Res. 2013; 41:8896–907.
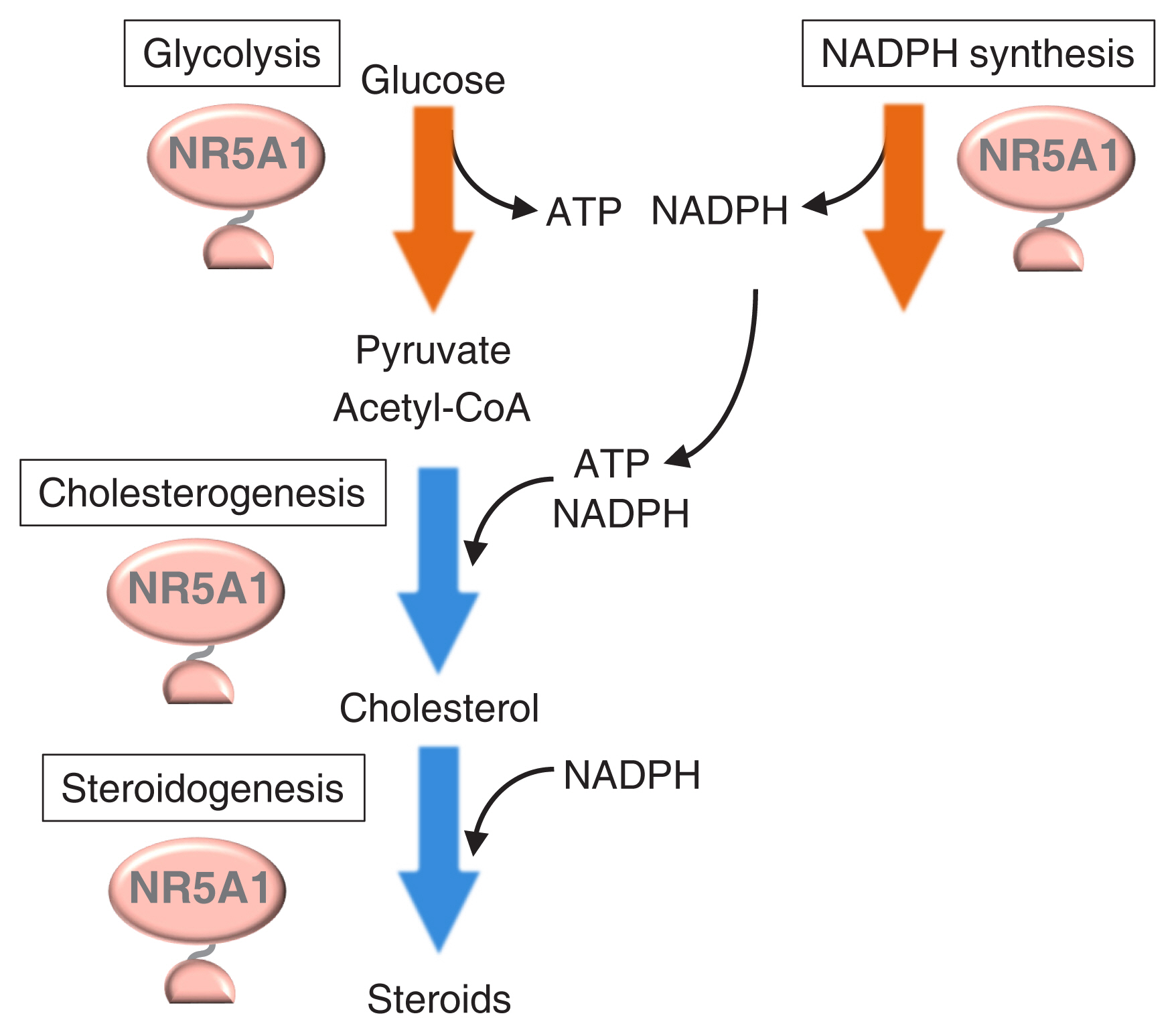
Article55. Baba T, Otake H, Sato T, Miyabayashi K, Shishido Y, Wang CY, et al. Glycolytic genes are targets of the nuclear receptor Ad4BP/SF-1. Nat Commun. 2014; 5:3634.
Article56. Ruggiero C, Doghman-Bouguerra M, Sbiera S, Sbiera I, Parsons M, Ragazzon B, et al. Dosage-dependent regulation of VAV2 expression by steroidogenic factor-1 drives adrenocortical carcinoma cell invasion. Sci Signal. 2017; 10:eaal2464.57. Li B, Baba T, Miyabayashi K, Sato T, Shima Y, Ichinose T, et al. Role of Ad4-binding protein/steroidogenic factor 1 in regulating NADPH production in adrenocortical Y-1 cells. Endocr J. 2017; 64:315–24.
Article58. Baba T, Otake H, Inoue M, Sato T, Ishihara Y, Moon JY, et al. Ad4BP/SF-1 regulates cholesterol synthesis to boost the production of steroids. Commun Biol. 2018; 1:18.
Article59. Sbiera S, Schmull S, Assie G, Voelker HU, Kraus L, Beyer M, et al. High diagnostic and prognostic value of steroidogenic factor-1 expression in adrenal tumors. J Clin Endocrinol Metab. 2010; 95:E161–71.
Article60. Hornstein I, Alcover A, Katzav S. Vav proteins, masters of the world of cytoskeleton organization. Cell Signal. 2004; 16:1–11.
Article61. Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988; 9:295–318.
Article62. Jinn S, Brandis KA, Ren A, Chacko A, Dudley-Rucker N, Gale SE, et al. snoRNA U17 regulates cellular cholesterol trafficking. Cell Metab. 2015; 21:855–67.
Article
- Full Text Links
- Actions
-
Cited
- CITED
-
- Close
- Share
- Similar articles
-
- The rare case of 46,XX testicular disorder of sex development carrying a heterozygous p.Arg92Trp variant in NR5A1
- Congenital Adrenal Agenesis Presented with Adrenal Insufficiency
- Coordination of Pharyngeal and Esophageal Phases of Swallowing
- Regulation of Stem Cell Fate by ROS-mediated Alteration of Metabolism
- Reactive Oxygen Species: Role in Pathophysiology, and Mechanism of Endogenous and Dietary Antioxidants during Oxidative Stress